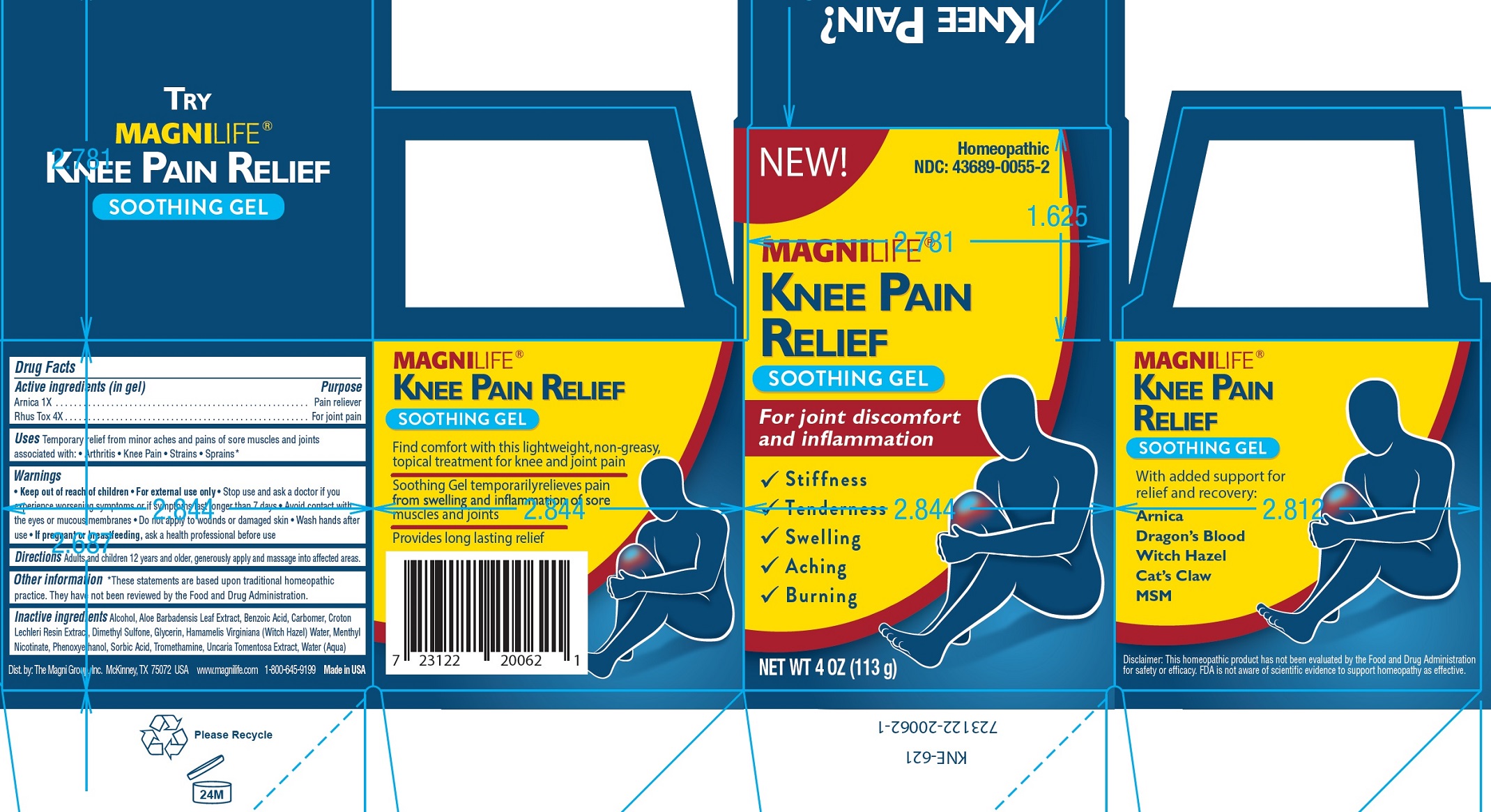
MAGNILIFE KNEE PAIN RELIEF GEL- arnica montana flower, toxicodendron pubescens leaf gel
The Magni Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active ingredients (in gel)
Arnica 1X
Rhus Tox 4X
Purpose
Pain reliever
For joint pain
Uses
Temporary relief from minor aches and pains of sore muscles and joints associated with: • Arthritis • Knee Pain • Strains • Sprains
Warnings
• For external use only
Keep out of reach of children
Stop use and ask a doctor
• if you experience worsening symptoms or if symptoms last longer than 7 days • Avoid contact with the eyes or mucous membranes • Do not apply to wounds or damaged skin • Wash hands after use
If pregnant or breastfeeding,
• ask a health professional before use
Directions
Adults and children 12 years and older, generously apply and massage into affected areas.
Other information
*These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
Inactive ingredients
Alcohol, Aloe Barbadensis Leaf Extract, Benzoic Acid, Carbomer, Croton Lechleri Resin Extract, Dimethyl Sulfone, Glycerin, Hamamelis Virginiana (Witch Hazel) Water, Menthyl Nicotinate, Phenoxyethanol, Sorbic Acid, Tromethamine, Uncaria Tomentosa Extract, Water (Aqua)
Package Labeling: