MENTHOLATUM PAIN RELIEF EXTRA STRENGTH- menthol gel
The Mentholatum Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
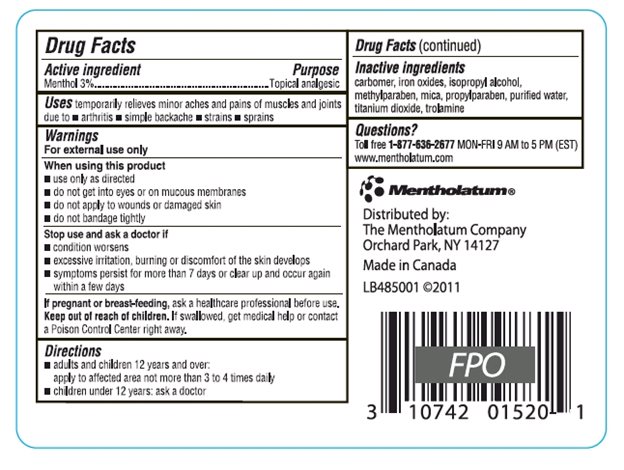
Drug Facts
Uses
temporarily relieves minor aches and pains of muscles and joints due to
- •
- simple backache
- •
- arthritis
- •
- strains
- •
- sprains
Warnings
For external use only
Keep Out of Reach of Children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- adults and children 12 years and over: apply to affected area not more than 3 to 4 times daily
- •
- children under 12 years: ask a doctor
Inactive ingredients
carbomer, iron oxides, isopropyl alcohol, methylparaben, mica, propylparaben, purified water, titanium dioxide, trolamine
| MENTHOLATUM PAIN RELIEF EXTRA STRENGTH
menthol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - The Mentholatum Company (002105757) |
Revised: 11/2016
Document Id: 937d0439-12a8-4f57-943e-3f5f9926463e
Set id: d7ea3cfc-dc14-4039-ac6b-7176dbe70cf5
Version: 2
Effective Time: 20161104
The Mentholatum Company