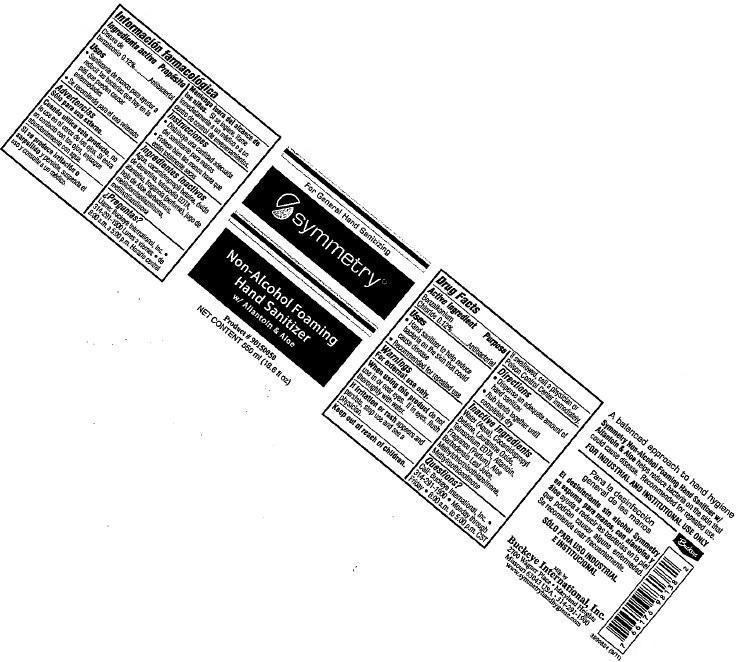
SYMMETRY NON-ALCOHOL FOAMING HAND SANITIZER WITH ALLANTOIN AND ALOE- benzalkonium chloride liquid
Project Cu, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
symmetry Non-Alcohol Foaming Hand Sanitizer with Allantoin & Aloe - 007
Uses
- Hand sanitizer to help reduce bacteria on the skin could cause disease
- Recommended for repeated use
Inactive ingredients
Water (Aqua), Cocamidopropyl Betaine, Lauramine Oxide, Tetrasodium EDTA, Allantoin, Fragrance (Parfum), Aloe Barbadensis Leaf Juice, Methylchloroisothiazolinone, Methylisothiazolinone
| SYMMETRY NON-ALCOHOL FOAMING HAND SANITIZER WITH ALLANTOIN AND ALOE
benzalkonium chloride liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Project Cu, Inc. (060555505) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Buckeye International, Inc. | 077132280 | manufacture(64305-007) | |
Revised: 11/2022
Document Id: edacbf12-f1d2-2650-e053-2a95a90a1302
Set id: d7be9725-4880-4a62-8f66-1d066914ad48
Version: 5
Effective Time: 20221117
Project Cu, Inc.