QUALITY CHOICE MUSCLE AND JOINT - menthol gel
Quality Home Products
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Menthol 2.5%
Uses
- For the timporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis and sprains
Warnings
For external use only.
-
DO NOT USE WITH A HEATING PAD
-
DO NOT BANDAGE TIGHTLY
-
DO NOT SWALLOW
- If swallowed, induce vomiting and call a physician. Keep away from eyes, mucous membranes, broken or irritated skin. If redness or irritation develops, pain lasts more than 10 days or with arthritis-like conditions in children under 12, do not use and call a physician.
KEEP AWAY FROM CHILDREN TO AVOID ACCIDENTAL POISONING.
Enter section text here
Directions
- Apply generously and gently massage until gel disappears
- Repeat 3 to 4 times daily
- Relief lasts for hours
For best results, squeeze tube from the bottom and flatten as you use product
Other information
-
store between 15° - 30°C (59° to 86°F)
Inactive ingredients
camphor, carbomer 940, potassium sorbate, isoprophyl alcohol, methylparaben, polysorbate 60, purified water, and trolamine
Package label
QC Muscle and Joint Vanishing Scent Gel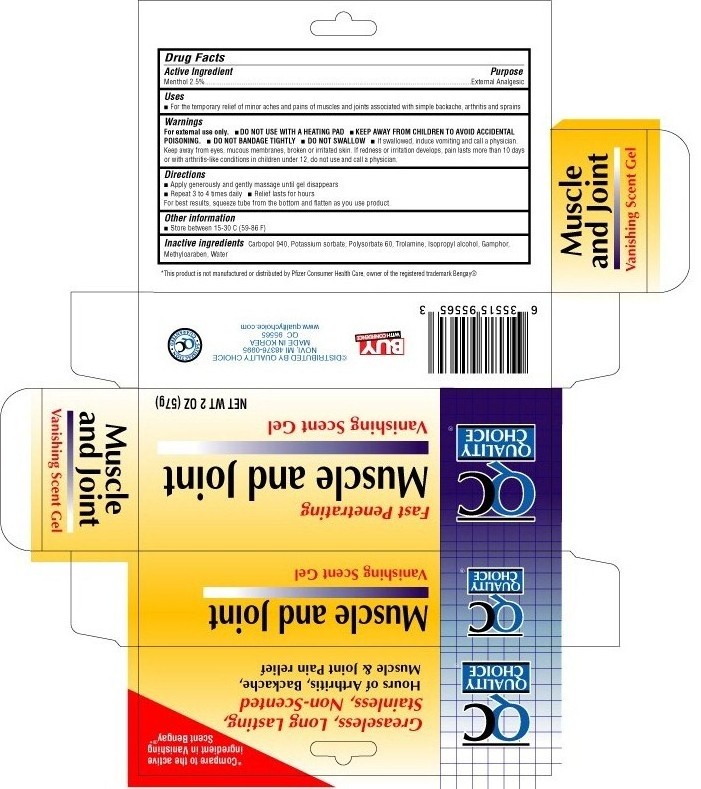
Purpose
External analgesic
