DESCRIPTION
Meprobamate is awhite powder with a characteristic odor and a bittertaste. It is slightly soluble in water, freely soluble in acetone andalcohol, and sparingly soluble in ether. The structural formula of meprobamate is:
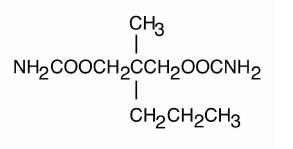
C9H18N2O4 M.W.218.25
Meprobamate Tablets USP 200 mg and 400 mg for oral administrationcontain the following inactive ingredients: microcrystalline cellulose,sodium starch glycolate, pre-gelatinized starch, colloidal silicondioxide, stearic acid and magnesium stearate.
CLINICAL PHARMACOLOGY
Meprobamate is a carbamate derivative which has been shown inanimal studies to have effects at multiple sites in the central nervoussystem including the thalamus and limbic system.
INDICATIONS AND USAGE
Meprobamate tablets are indicated for the management of anxietydisorders or for the short-term relief of the symptoms of anxiety.Anxiety or tension associated with the stress of everyday life usuallydo not require treatment with an anxiolytic.
The effectiveness of meprobamate tablets in long-term use, that is,more than 4 months, has not been assessed by systematic clinicalstudies. The physician should periodically reassess the usefulness ofthe drug for the individual patient.
CONTRAINDICATIONS
Acute intermittent porphyria as well as allergic or idiosyncraticreactions to meprobamate or related compounds such ascarisoprodol, mebutamate, tybamate, or carbromal.
WARNINGS
Drug Dependence
Physical dependence, psychological dependence, and abuse haveoccurred. When chronic intoxication from prolonged use occurs, itusually involves ingestion of greater than recommended doses and ismanifested by ataxia, slurred speech, and vertigo. Therefore, carefulsupervision of dose and amounts prescribed is advised, as well asavoidance of prolonged administration, especially for alcoholics andother patients with a known propensity for taking excessive quantitiesof drugs.
Sudden withdrawal of the drug after prolonged and excessive use mayprecipitate recurrence of pre-existing symptoms such as anxiety,anorexia, insomnia, or withdrawal reactions such as vomiting, ataxia,tremors, muscle twitching, confusional states, hallucinosis, andrarely, convulsive seizures. Such seizures are more likely to occur inpersons with central nervous system damage or pre-existent or latentconvulsive disorders. Onset of withdrawal symptoms occurs usuallywithin 12 to 48 hours after discontinuation of meprobamate;symptoms usually cease within the next 12 to 48 hours.
When excessive dosage has continued for weeks or months, dosageshould be reduced gradually over a period of one or two weeks ratherthan abruptly stopped. Alternatively, a long-acting barbiturate may besubstituted, then gradually withdrawn.
Potentially Hazardous Tasks
Patients should be warned that meprobamate may impair the mentaland/or physical abilities required for performance of potentiallyhazardous tasks such as driving or operating machinery.
Additive Effects
Since the effects of meprobamate and alcohol or meprobamate andother CNS depressants or psychotropic drugs may be additive,appropriate caution should be exercised with patients who take morethan one of these agents simultaneously.
Usage in Pregnancy and Lactation
An increased risk of congenital malformations associated with theuse of minor tranquilizers (meprobamate, chlordiazepoxide anddiazepam) during the first trimester of pregnancy has beensuggested in several studies. Because use of these drugs is rarelya matter of urgency, their use during this period should almostalways be avoided. The possibility that a woman of childbearingpotential may be pregnant at the time of institution of therapyshould be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant theyshould communicate with their physician about the desirability ofdiscontinuing the drug.
Meprobamate passes the placental barrier. It is present both inumbilical cord blood at or near maternal plasma levels and inbreast milk of lactating mothers at concentrations two to four timesthat of maternal plasma. When use of meprobamate iscontemplated in breastfeeding patients, the drug's higherconcentration in breast milk as compared to maternal plasmashould be considered.
PRECAUTIONS
The lowest effective dose should be administered, particularly toelderly and/or debilitated patients, in order to preclude oversedation.
The possibility of suicide attempts should be considered and the leastamount of drug feasible should be dispensed at anyone time.
Meprobamate is metabolized in the liver and excreted by the kidney; toavoid its excess accumulation, caution should be exercised inadministration to patients with compromised liver or kidney function.
Meprobamate occasionally may precipitate seizures in epilepticpatients.
Geriatric Use
Clinical studies of meprobamate tablets did not include sufficientnumbers of subjects aged 65 and over to determine whether theyrespond differently from younger subjects. Other reported clinicalexperience has not identified differences in responses between theelderly and younger patients. In general, dose selection for an elderlypatient should be cautious, usually starting at the low end of thedosing range, reflecting the greater frequency of decreased hepatic,renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Central Nervous System
Drowsiness, ataxia, dizziness, slurred speech, headache, vertigo,weakness, paresthesias, impairment of visual accommodation,euphoria, overstimulation, paradoxical excitement, fast EEG activity.
Cardiovascular
Palpitation, tachycardia, various forms of arrhythmia, transient ECGchanges, syncope; also hypotensive crisis (including one fatal case).
Allergic or Idiosyncratic
Allergic or idiosyncratic reactions are usually seen within the period ofthe first to fourth dose in patients having had no previous contact withthe drug. Milder reactions are characterized by an itchy, urticarial, orerythematous maculopapular rash which may be generalized orconfined to the groin. Other reactions have included leukopenia, acutenonthrombocytopenic purpura, petechiae, ecchymoses, eosinophilia,peripheral edema, adenopathy, fever, fixed drug eruption with crossreaction to carisoprodol, and cross sensitivity betweenmeprobamate/mebutamate and meprobamate/carbromal.
More severe hypersensitivity reactions, rarely reported, includehyperpyrexia, chills, angioneurotic edema, bronchospasm, oliguriaand anuria. Also, anaphylaxis, erythema multiforme, exfoliativedermatitis, stomatitis, proctitis, Stevens-Johnson syndrome andbullous dermatitis, including one fatal case of the latter followingadministration of meprobamate in combination with prednisolone.
In case of allergic or idiosyncratic reactions to meprobamate,discontinue the drug and initiate appropriate symptomatic therapy,which may include epinephrine, antihistamines, and in severe cases,corticosteroids. In evaluating possible allergic reactions, also considerallergy to excipients.
Hematologic
(See also Allergic or Idiosyncratic). Agranulocytosis and aplasticanemia have been reported. These cases rarely were fatal. Rare casesof thrombocytopenic purpura have been reported.
OVERDOSAGE
Suicidal attempts with meprobamate have resulted in drowsiness,lethargy, stupor, ataxia, coma, shock, vasomotor, and respiratorycollapse. Some suicidal attempts have been fatal.
The following data on meprobamate tablets have been reported in theliterature and from other sources. These data are not expected tocorrelate with each case (considering factors such as individual susceptibility and length of time from ingestion to treatment), butrepresent the usual ranges reported.
Acute simple overdose
(meprobamate alone): Death has beenreported with ingestion of as little as 12 g meprobamate and survivalwith as much as 40 g.
Blood levels
0.5-2 mg% represents the usual blood level range of meprobamateafter therapeutic doses. The level may occasionally be as high as 3mg%.
3-10 mg% usually corresponds to findings of mild to moderatesymptoms of overdosage, such as stupor or light coma.
10-20 mg% usually corresponds to deeper coma, requiring moreintensive treatment. Some fatalities occur.
At levels greater than 20 mg%, more fatalities than survivals can beexpected.
Acute combined overdose
(meprobamate with alcohol or other CNSdepressants or psychotropic drugs): Since effects can be additive, ahistory of ingestion of a low dose of meprobamate plus any of thesecompounds (or of a relative low blood or tissue level) cannot be usedas a prognostic indicator.
In cases where excessive doses have been taken, sleep ensues rapidlyand blood pressure, pulse, and respiratory rates are reduced to basallevels. Any drug remaining in the stomach should be removed andsymptomatic therapy given. Should respiration or blood pressurebecome compromised, respiratory assistance, central nervous systemstimulants, and pressor agents should be administered cautiously asindicated. Meprobamate is metabolized in the liver and excreted by thekidney. Diuresis, osmotic (mannitol) diuresis, peritoneal dialysis, andhemodialysis have been used successfully. Careful monitoring ofurinary output is necessary and caution should be taken to avoidoverhydration. Relapse and death, after initial recovery, have beenattributed to incomplete gastric emptying and delayed absorption.Meprobamate can be measured in biological fluids by two methods:colorimetric (Hoffman, A.J. and Ludwig, B.J.: J Amer Pharm Assn 48:740, 1959) and gas chromatographic (Douglas, J.F. et al: Anal Chern39: 956, 1967).
DOSAGE AND ADMINISTRATION
Meprobamate Tablets USP: The usual adult daily dosage is 1200 mgto 1600 mg, in three or four divided doses; adaily dosage above 2400mg is not recommended. The usual daily dosage for children ages sixto twelve years is 200 mg to 600 mg, in two or three divided doses.
Not recommended for children under age 6 (see Usage in Children).
HOW SUPPLIED
Meprobamate Tablets USP 200 mg are white, round, biconvex tabletsdebossed with I and 7 on one side and bisect on the other.
Supplied in bottles of 100 and 1000.
Bottles of 100: NDC 55111-640-01
Bottles of 1000: NDC 55111-640-10
Meprobamate Tablets USP 400 mg are white, round, biconvex tabletsdebossed with I and 4 on one side and bisect on the other.
Supplied in bottles of 100 and 500.
Bottles of 100: NDC 55111-641-01
Bottles of 500: NDC 55111-641-05
Dispense in well-closed container with child-resistant closure.
Store at 20°-25°C (68°-77°F) [see USP Controlled RoomTemperature].
Manufactu red by:
InvaGen Pharmaceuticals, Inc
Hauppauge, NY 11788
Manufactured for:
Dr. Reddy's Laboratories Inc.,
Bridgewater, NJ 08807 USA
Rev: 05/09