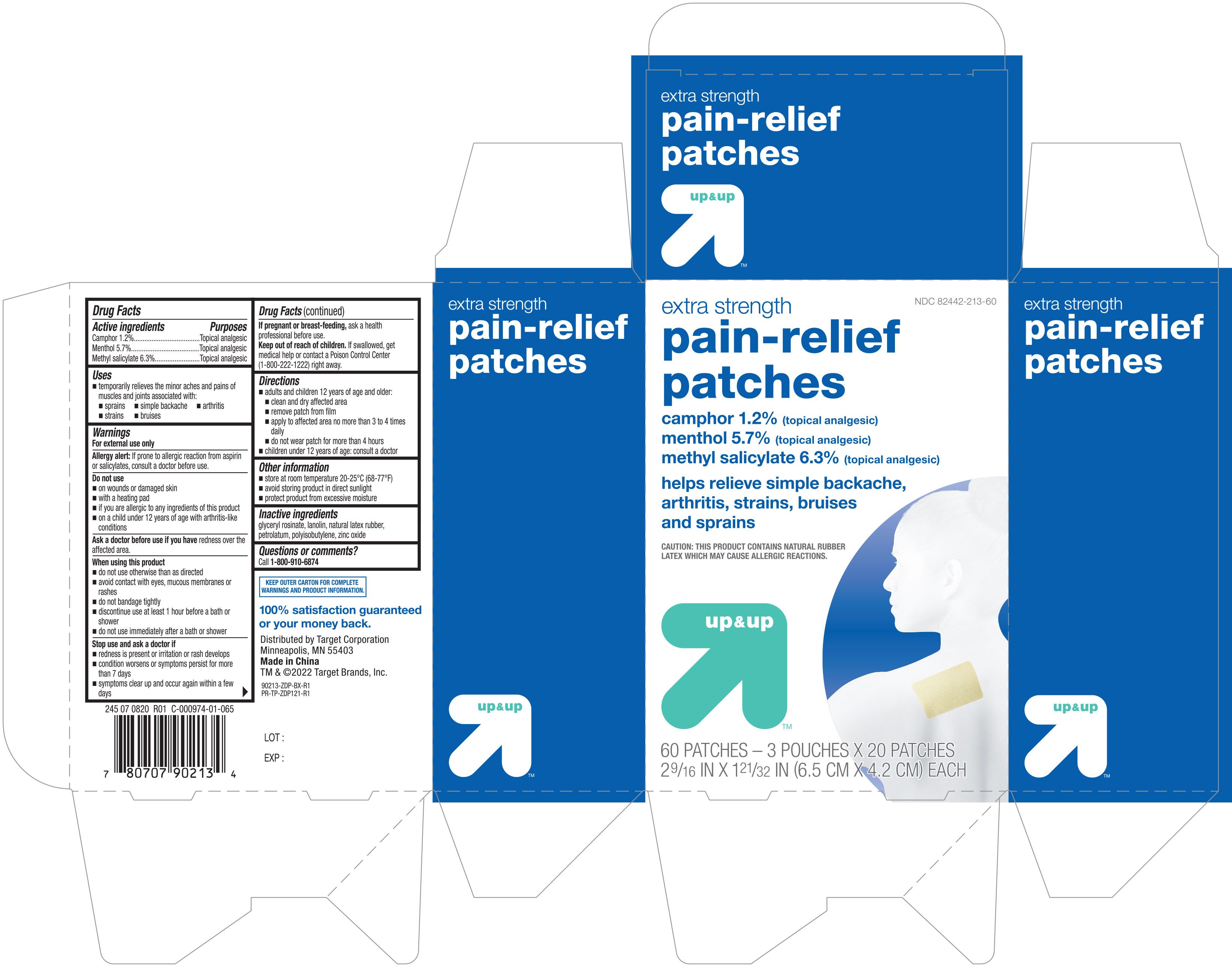
Active ingredients Purposes
Camphor 1.2%....................................................Topical analgesic
Menthol 5.7%......................................................Topical analgesic
Methyl Salicylate 6.3%........................................Topical analgesic
Uses
temporarily relieves the minor aches and pains of muscles and joints associated with:
- sprains
- simple backache
- arthirtis
- strains
- bruises
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use
Do not use
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
- on a child under 12 years of age with arthritis-like conditions
When using this product
- do not use otherwise than as directed
- avoid contact with eyes, mucous membranes or rashes
- do not bandage tightly
- discontinue use at least 1 hour before a bath or shower
- do not use immediately after a bath or shower
Stop use and ask a doctor if
- redness is present or irritation or rash develops
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away
Directions
- adults and children 12 years of age and older:
- clean and dry affected area
- remove patch from film
- apply to affected area no more than 3 to 4 times daily
- do not wear patch for more than 4 hours
- children under 12 years of age: consult a doctor
Other information
- store at room temperature 20-25°C (68-77°F)
- avoid storing product in direct sunlight
- protect product from excessive moisture