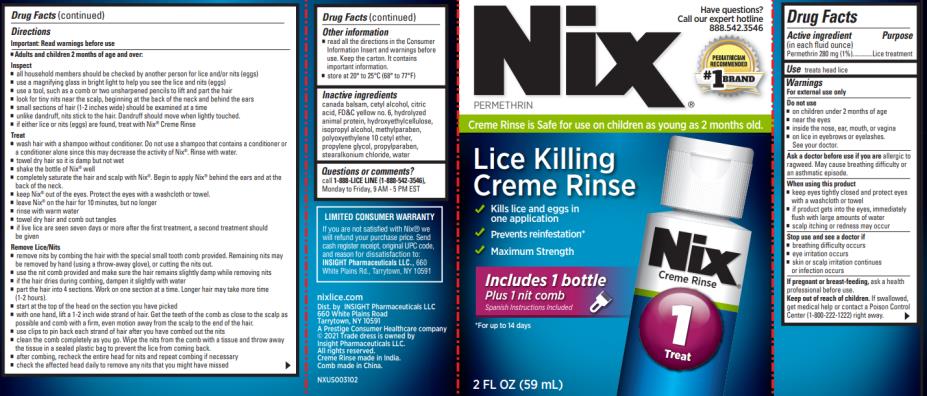
Warnings
For external use only
Do not use
- on children under 2 months of age
- near the eyes
- inside the nose, ear, mouth, or vagina
- on lice in eyebrows or eyelashes. See your doctor.
Ask a doctor before use if you are
allergic to ragweed. May cause breathing difficulty or an asthmatic episode.
When using this product
- keep eyes tightly closed and protect eyes with a washcloth or towel
- if product gets into the eyes, immediately flush with large amounts of water
- scalp itching or redness may occur
Directions
Important: Read warnings before use
■ Adults and children 2 months of age and over:
Inspect
- all household members should be checked by another person for lice and/or nits (eggs)
- use a magnifying glass in bright light to help you see the lice and nits (eggs)
- use a tool, such as a comb or two unsharpened pencils to lift and part the hair
- look for tiny nits near the scalp, beginning at the back of the neck and behind the ears
- small sections of hair (1-2 inches wide) should be examined at a time
- unlike dandruff, nits stick to the hair. Dandruff should move when lightly touched.
- if either lice or nits (eggs) are found, treat with Nix® Creme Rinse
Treat
- wash hair with a shampoo without conditioner. Do not use a shampoo that contains a conditioner or a conditioner alone since this may decrease the activity of Nix®. Rinse with water.
- towel dry hair so it is damp but not wet
- shake the bottle of Nix® well
- completely saturate the hair and scalp with Nix®. Begin to apply Nix® behind the ears and at the back of the neck.
- keep Nix® out of the eyes. Protect the eyes with a washcloth or towel.
- leave Nix® on the hair for 10 minutes, but no longer
- rinse with warm water
- towel dry hair and comb out tangles
- if live lice are seen seven days or more after the first treatment, a second treatment should be given
Remove Lice/Nits
- remove nits by combing the hair with the special small tooth comb provided. Remaining nits may be removed by hand (using a throw-away glove), or cutting the nits out.
- use the nit comb provided and make sure the hair remains slightly damp while removing nits
- if the hair dries during combing, dampen it slightly with water
- part the hair into 4 sections. Work on one section at a time. Longer hair may take more time (1-2 hours).
- start at the top of the head on the section you have picked
- with one hand, lift a 1-2 inch wide strand of hair. Get the teeth of the comb as close to the scalp as possible and comb with a firm, even motion away from the scalp to the end of the hair.
- use clips to pin back each strand of hair after you have combed out the nits
- clean the comb completely as you go. Wipe the nits from the comb with a tissue and throw away the tissue in a sealed plastic bag to prevent the lice from coming back.
- after combing, recheck the entire head for nits and repeat combing if necessary
- check the affected head daily to remove any nits that you might have missed
Other information
- read all the directions in the Consumer Information Insert and warnings before use. Keep the carton. It contains important information.
- store at 20° to 25°C (68° to 77°F)