FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
NUWIQ is a recombinant antihemophilic factor [blood coagulation factor VIII (Factor VIII)] indicated in adults and children with Hemophilia A for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
- Routine prophylaxis to reduce the frequency of bleeding episodes
NUWIQ is not indicated for the treatment of von Willebrand disease.
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution
2.1 Dose
- Each vial of NUWIQ is labeled with the actual Factor VIII potency expressed in international units (IU). One IU of Factor VIII activity is defined by the quantity of Factor VIII in one mL of normal human pooled plasma. Calculation of the required dose of Factor VIII is based on the empirical finding that 1 IU Factor VIII per kg body weight raises the plasma Factor VIII activity by approximately 2% of normal activity or 2 IU/dL when assessed using the one stage clotting assay. Use the following formulae to determine the required dose:
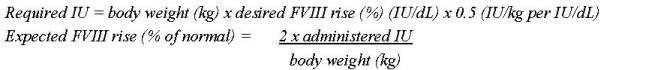
- Dose and duration of therapy depend on the severity of the Factor VIII deficiency, the location and extent of the bleeding, FVIII level, and the patient’s clinical condition.
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing NUWIQ for the on-demand treatment and control of bleeding episodes is provided in Table 1 . Selected dosing regimen should maintain plasma Factor VIII activity levels at or above the plasma levels (in % of normal or in IU/dL) outlined in the table.
Table 1: Dosing for Treatment and Control of Bleeding Episodes
| Type of Bleeding Episodes |
Required peak post-infusion Factor VIII activity
(% of normal or IU/dL) | Frequency of Dosing (hours) | Duration of Therapy (days) |
|
Minor:
Superficial muscle or soft tissue and oral bleeds | 20–40 | 12-24 | At least 1 day, until the bleeding episode is resolved. |
|
Moderate to Major:
Hemorrhage into muscles, into oral cavity, hemarthrosis, known trauma | 30–60 | 12-24 | For 3–4 days or more until bleeding episode is resolved. |
| Life-threatening: Intracranial, intra-abdominal, gastro-intestinal or intrathoracic bleeds, central nervous system bleeds, bleeding in retropharyngeal spaces or iliopsoas sheath, eyes/retina, fractures or head trauma | 60–100 | 8-24 | Until bleeding risk is resolved. |
Perioperative Management
A guide for dosing NUWIQ during surgery (perioperative management) is provided in Table 2 . Dosing should aim at maintaining a plasma Factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in the table.
Table 2: Dosing for Perioperative Management
| Type of Surgery |
Required post-infusion Factor VIII activity
(% of normal or IU/dL) | Frequency of Doses (hours) | Duration of Therapy (days) |
|
Minor
including tooth extraction |
30–60 (pre- and post-operative) | 24 | At least 1 day, until healing is achieved. |
|
Major
Intracranial, intra-abdominal, or joint-replacement therapy |
80–100 (pre- and post-operative) | 8-24 | Until adequate wound healing, then continue therapy for at least another 7 days to maintain a Factor VIII activity of 30% to 60% (IU/dL). |
Routine Prophylaxis
A guide for dosing NUWIQ for routine prophylaxis to reduce the frequency of bleeding is provided in Table 3 . Exact dosing should be defined by the patient’s clinical status and response.
Table 3: Dosing for Routine Prophylaxis
| Subjects | Dose (IU/kg) | Frequency of infusions |
| Adults and adolescents [12 - 17 yrs] | 30 - 40 | Every other day |
| Children [2 - 11 yrs] | 30 - 50 | Every other day or three times per week |
A regimen may be further individually adjusted to less or more frequent dosing at the discretion of the treating physician.
2.2 Preparation and Reconstitution
NUWIQ package contents:
- single-use vial of NUWIQ concentrate
- pre-filled syringe containing 2.5 mL Sterile Water for Injection
- vial adapter
- butterfly needle
- two alcohol swabs.
- Always work on a clean surface and wash your hands before performing the procedure.
- Allow the vial of NUWIQ and the pre-filled syringe to come to room temperature.
- Remove the plastic flip-top cap from the NUWIQ vial to expose the rubber stopper. (Figure A).
- Wipe the top of the vial with an alcohol swab and allow the rubber stopper of the vial to dry.
- Peel back the paper cover from the vial adapter package revealing the adapter spike without removing the adapter from the package (Figure B).
- With the concentrate vial on an even surface, insert the adapter spike into the rubber stopper. The adapter snaps to the vial when done (Figure C).
- Peel back the paper cover from the pre-filled syringe package. Connect plunger rod attaching the threaded end of the plunger rod to the solvent syringe, turning clockwise until a slight resistance is felt (Figure D). Avoid contact with shaft.
- Break off the tamper-proof plastic tip from the syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip (Figure E).
- Remove the adapter packaging and connect the syringe to the vial adapter by turning clockwise until resistance is felt (Figure F).
- Slowly inject all liquid from syringe into the concentrate vial (Figure G).
- Without removing the syringe, dissolve the concentrate powder in the vial by gently moving or swirling a few times. DO NOT SHAKE. Wait until all the powder dissolves completely.
- Inspect the final solution for particles. The solution should be clear, colorless, and free from visible particles. Do not use if solution is cloudy or has particulate matter.
- Turn the vial and syringe upside down (still attached).
- Slowly withdraw the solution into the syringe. Make sure that all liquid is transferred to the syringe (Figure H).
- Detach the filled syringe from the vial adapter by turning counter clockwise.
Do not refrigerate the solution after reconstitution. Use the solution within 3 hours after reconstitution. If solution is not used within this time period, close the filled syringe with the tamper-proof plastic tip, and discard.
2.3 Administration
For intravenous use after reconstitution only
- Inspect the reconstituted NUWIQ solution for visible particulate matter and discoloration prior to administration. Do not use if particulate matter or discoloration is observed.
- Do not administer NUWIQ in the same tubing or container as other medications.
- Clean the chosen injection site with an alcohol swab.
- Attach the provided infusion set to the syringe. Insert the needle of the infusion set into the chosen vein.
- Perform intravenous bolus infusion. The rate of administration should be determined by patient’s comfort level, at a maximum rate of 4 mL per minute.
- After infusing NUWIQ, remove and properly discard the infusion set. After the infusion, remove the peel-off label containing the batch number from the factor concentrate vial and place it in the log book for record keeping.
3 DOSAGE FORMS AND STRENGTHS
NUWIQ is available as a white, sterile, non-pyrogenic, lyophilized powder for reconstitution in single-use vials containing nominally 250, 500, 1000, 1500, 2000, 2500, 3000, or 4000 IU Factor VIII potency. The actual Factor VIII potency is labeled on each NUWIQ vial.
4 CONTRAINDICATIONS
NUWIQ is contraindicated in patients who have manifested life-threatening hypersensitivity reactions, including anaphylaxis, to the product or its components.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, are possible with NUWIQ. Early signs of hypersensitivity reactions that can progress to anaphylaxis may include angioedema, chest tightness, dyspnea, wheezing, urticaria, or pruritus. Immediately discontinue administration and initiate appropriate treatment if hypersensitivity reactions occur.
5.2 Neutralizing Antibodies
The formation of neutralizing antibodies (inhibitors) to Factor VIII can occur following the administration of NUWIQ. Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests. If the plasma Factor VIII level fails to increase as expected, or if bleeding is not controlled after NUWIQ administration, suspect the presence of an inhibitor (neutralizing antibody) [see Warnings and Precautions (5.3) ] .
5.3 Monitoring and Laboratory Tests
- Monitor plasma Factor VIII activity by performing a validated test (e.g., one stage clotting assay), to confirm that adequate Factor VIII levels have been achieved and maintained [see Dosage and Administration (2.1) ] .
- Monitor for the development of Factor VIII inhibitors. Perform a Bethesda inhibitor assay if expected Factor VIII plasma levels are not attained, or if bleeding is not controlled with the expected dose of NUWIQ. Use Bethesda Units (BU) to report inhibitor levels.
6 ADVERSE REACTIONS
The most common adverse reactions (>5% of subjects) reported in clinical trials were upper respiratory tract infection, headache, fever, cough, lower respiratory tract infection, rhinitis, chills, abdominal pain, arthralgia, anemia, and pharyngitis.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rate in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety profile of NUWIQ was evaluated in seven prospective, open-label clinical studies in previously treated patients (PTPs - exposed to a Factor VIII containing product for ≥150 exposure days (EDs) in the case of adolescents and adults or ≥50 EDs in the case of subjects below 12 years of age) with severe Hemophilia A (Factor VIII ≤ 1%). Subjects who had a history of detectable Factor VIII inhibitor, severe liver or kidney disease, were not immune competent (CD4+ count <200/µL), or scheduled to receive immunomodulating drugs, were excluded.
Across all clinical studies, 190 patients were stratified, among them, 129 were adults, 3 were adolescents between 12 and 17 years old, and 58 were pediatric patients between 2 and 11 years old. A total of 182 (95.8%) subjects were treated for at least 180 days. Collectively, patients received between 24,005 and 2.12 million IU (555 to 34,713 IU/kg) from 14 to 918 infusions over a period of 14 to 896 exposure days. An exposure day was defined as any day on which at least one infusion was started.
With a total of 43,264 infusions over 42,808 EDs, adverse reactions reported in at least 5 of the 190 subjects (2.6%) were upper respiratory tract infection (22%), headache (11%), fever (10%), cough (9.5%), lower respiratory tract infection (8.4%), rhinitis (7.9%), chills (6.8%), abdominal pain (5.3%), arthralgia (5.3%), pharyngitis (5.3%), diarrhea (4.7%), varicella (4.2%), device-related problems (4.2%), back pain (3.7%), extremity pain (3.7%), toothache (3.2%), vomiting (3.2%), head injury (2.6%), injury (2.6%), rash (2.6%), tonsillitis (2.6%), and tooth abscess (2.6%). Notably, dizziness was reported in 4 subjects (2.1%); dyspnea, insomnia, and malaise in 2 subjects (1.1%) each; and injection site pain, injection site inflammation, benign renal neoplasm, deterioration in neurological function/behavior, and syncope in 1 subject (0.5%) each.
Considering only the pediatric cohort of 59 subjects (ages 2-12 years at initial enrollment), 48 of whom continued participation in a long-term extension study, 26,311 infusions totalling 1,019,528 IU/kg NUWIQ were administered. The most commonly reported adverse reactions (>10% of the 59 subjects) were upper respiratory tract infection (49%), lower respiratory tract infection (24%), cough (20%), fever (20%), rhinitis (19%), pharyngitis (19%), chills (15%), varicella (14%), headache (12%), abdominal pain (10%), and extremity pain (10%). Dizziness was reported in 2 subjects (3.4%), while deterioration in neurological function/behavior, insomnia, and syncope were each reported in 1 subject (1.7%).
6.2 Immunogenicity
All clinical trial subjects (N = 190) were monitored for neutralizing antibodies (inhibitors) to Factor VIII by the modified Bethesda assay using blood samples obtained prior to the first infusion of NUWIQ in all studies, at defined intervals (at ED 10 to 15, at three months, and every further three months) in five studies, and every three months in one study and at the completion visit in all studies. No subject developed neutralizing antibodies to Factor VIII.
Non-neutralizing anti-Factor VIII antibodies (without inhibitory activity as measured by the modified Bethesda assay) were reported in four of 135 patients tested, giving a rate of 3%. Three of four subjects had pre-existing non-neutralizing antibodies prior to exposure with NUWIQ. The binding antibodies were transient in two of these three subjects. In one subject who was tested negative at screening, the non-neutralizing antibody was measured once at study end.
In a clinical trial, which enrolled 110 previously untreated patients (age range 0-146 months, no previous treatment with FVIII concentrates or other blood products containing FVIII was allowed), 108 had evaluable data post-treatment with NUWIQ. The primary endpoint of the study, inhibitor formation, was realized in 28 of 105 patients (26.7%; 95% CI: 18.5-36.2) with at least one inhibitor analysis after ED1. Seventeen patients (16.2%; 95% CI: 9.7-24.7) developed high-titer inhibitors and 11 (10.5%; 95% CI: 5.3-18) developed low-titer inhibitors, 5 of whom had transient inhibitors. Of the 28 patients who developed an inhibitor, 25 did so with ≤20 EDs prior to detection. All inhibitors developed within 34 ED.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to NUWIQ with the incidence of antibodies to other products may be misleading.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with NUWIQ use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with NUWIQ. It is not known whether NUWIQ can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. NUWIQ should be given to a pregnant woman only if clearly needed. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of NUWIQ in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NUWIQ and any potential adverse effects on the breastfed infant from NUWIQ or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of NUWIQ were assessed in a study in 59 previously treated pediatric patients (58 patients 2 to 11 years old) who received at least one dose of NUWIQ for routine prophylaxis. The safety and efficacy in routine prophylaxis and on-demand treatment of bleeding episodes are comparable between children and adults [see Clinical Trial Experience (6) and Clinical Studies (14) ] . The pediatric pharmacokinetic data of NUWIQ were obtained in 29 children between 2 and 5 years of age and 30 children between 6 and ≤12 years of age. Half-life (T 1/2 ) and incremental in vivo recovery (IVR) are lower in children than in adults and systemic drug clearance is substantially higher in the pediatric age group 2 to 5 yrs compared to adults [see Clinical Pharmacology (12.3) ] . Higher doses and/or a more frequent dosing schedule for prophylactic treatment should be considered in pediatric patients aged 2 to 5 yrs.
The safety and efficacy of NUWIQ were assessed in a study in 110 previously untreated pediatric patients (PUPs, age range 0-146 months). Of 110 PUPs, 108 had evaluable data and were investigated to determine the immunogenicity [see Immunogenicity (6.2)] of NUWIQ, the primary endpoint. Secondary endpoints were efficacy during prophylaxis, on-demand, and surgical prophylaxis and safety and tolerability [see Adverse Reactions (6) ].
11 DESCRIPTION
NUWIQ, Antihemophilic Factor (Recombinant), is a sterile, non-pyrogenic, lyophilized powder for reconstitution for intravenous injection. The product is supplied in single-use vials containing nominal Factor VIII potencies of 250, 500, 1000, 1500, 2000, 2500, 3000 or 4000 IU. When reconstituted with 2.5 mL of solvent (Sterile Water for Injection), the respective nominal concentrations are 100, 200, 400, 600, 800, 1000, 1200 or 1600 IU/mL. The reconstituted product contains the following excipients per mL: 18 mg sodium chloride, 5.4 mg sucrose, 5.4 mg L-arginine hydrochloride, 0.3 mg calcium chloride dihydrate, 1.2 mg poloxamer 188, and 1.2 mg sodium citrate dihydrate. The concentration of each of the excipients is the same for all potencies. NUWIQ contains no preservatives. Each vial of NUWIQ is labeled with the actual Factor VIII potency expressed in IU determined using one-stage clotting assay, using a reference material calibrated against a World Health Organization (WHO) International Standard for Factor VIII concentrates. One IU, as defined by the WHO standard for human Factor VIII concentrates, is approximately equal to the level of Factor VIII activity in 1 mL of fresh pooled, normal, human plasma. The mean specific activity of NUWIQ is 8124 IU/mg total protein.
B-domain deleted recombinant coagulation Factor VIII (BDD-rFVIII) is the active ingredient in NUWIQ. BDD-rFVIII is a recombinant glycoprotein (a heterodimer) with an approximate molecular mass of 170 kDa, comprising the Factor VIII domains A1-A2 (so-called heavy chain of ~90 kDa) and A3-C1-C2 (so-called light chain of ~80 kDa), whereas the B-domain, present in the full-length plasma-derived Factor VIII, has been deleted. The purified protein consists of 1440 amino acids. The amino acid sequence is comparable to the B-domain deleted form of human plasma Factor VIII (90 + 80 kDa).
BDD-rFVIII is produced by recombinant DNA technology in genetically modified human embryonic kidney (HEK) 293F cells with no animal or human derived materials added during the manufacturing process or to the final product. As NUWIQ is produced using a human cell-line, it contains post-translational modifications comparable to human plasma-derived Factor VIII and is devoid of Neu5Gc or α-1,3-Gal epitopes that may be present in products produced in animal cells. Furthermore, BDD-rFVIII is fully sulfated at Tyr1680. The active substance is concentrated and purified by a series of chromatography steps, which also includes two dedicated viral clearance steps: solvent/detergent (S/D) treatment for virus inactivation and 20 nm nanofiltration for removal of viruses.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
NUWIQ temporarily replaces the missing clotting Factor VIII that is needed for effective hemostasis.
12.2 Pharmacodynamics
Hemophilia A is a bleeding disorder characterized by a deficiency of functional coagulation Factor VIII, resulting in a prolonged plasma clotting time as measured by the activated partial thromboplastin time (aPTT) assay. Treatment with NUWIQ normalizes the aPTT over the effective dosing period.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of NUWIQ were evaluated in an open-label, multi-center clinical study of 22 (20 adults and 2 adolescents) previously treated patients (PTPs) with severe Hemophilia A. The PK parameters ( Table 4 ) were based on plasma Factor VIII activity measured by the one-stage clotting assay after a single intravenous infusion of a 50 IU/kg dose.
The PK profile obtained after 6 months of repeated dosing was comparable with the PK profile obtained after the first dose.
Table 4: Pharmacokinetic Parameters of NUWIQ in 22 PTP Adults/Adolescents (Dose: 50 IU/kg)
| PK Parameters | Mean ± SD |
| AUC (h·IU/mL) | 18.0 ± 5.6 |
| AUC norm (h·IU/mL/(IU/kg)) | 0.4 ± 0.1 |
| C maxnorm (IU/mL/(IU/kg)) | 0.022 ± 0.003 |
| T 1/2 (h) | 17.1 ± 11.2* |
| IVR (%/IU/kg) | 2.1 ± 0.3 |
| MRT (h) | 22.5 ± 14.2 |
| CL (mL/h/kg) | 3.0 ± 1.0 |
| V ss (mL/kg) | 59.8 ± 19.8 |
AUC = Area under the curve (Factor VIII:C); AUCnorm = AUC divided by the dose; Cmaxnorm = Maximal plasma concentration divided by the dose; CL = Clearance; Factor VIII:C = Factor VIII coagulation activity; IVR = Incremental in vivo recovery; MRT = Mean residence time; PK = Pharmacokinetics; SD = Standard deviation; T1/2 = Terminal half-life; Vss = Volume of distribution at steady state; *Median, lower/upper quartile: 13.7, 12.0/17.5
Pediatric Pharmacokinetics
PK of pediatric patients is presented in Table 5 for the age groups 2 to 5 years and 6 to 12 years. They were based on plasma Factor VIII activity measured by the one-stage clotting assay after a single intravenous infusion of 50 IU/kg dose. Compared to adults and adolescents, IVR and T 1/2 were lower and systemic drug clearance (based on per kg bodyweight) was substantially higher in children 2 to 5 yr of age.
IVR analysis after 3 and 6 months of prophylactic treatment yielded comparable results with the IVR after the first dose.
As in the adult population, similar PK values were obtained using the chromogenic and the one-stage assay. The values in Table 5 reflect those obtained using the one-stage assay.
Table 5: Pharmacokinetic Parameters of NUWIQ in 26 PTP Children Age 2 to 5 Years and 6 to 12 Years (Dose: 50 IU/kg)
| PK Parameters |
2 to 5 years (N = 13)
Mean ± SD |
6 to ≤12 years (N = 13)
Mean ± SD |
| AUC (h·IU/mL) | 10.1 ± 4.6 | 11.8 ± 2.7 |
| AUC norm (h·IU/mL/(IU/kg)) | 0.2 ± 0.1 | 0.3 ± 0.1 |
| C maxnorm (IU/mL/(IU/kg)) | 0.016 ± 0.002 | 0.017 ± 0.004 |
| T 1/2 (h) | 11.9 ± 5.4 * | 13.1 ± 2.6 # |
| IVR (%/IU/kg) | 1.6 ± 0.2 | 1.6 ± 0.4 |
| MRT (h) | 15.1 ± 7.4 | 16.5 ± 2.9 |
| CL (mL/h/kg) | 5.4 ± 2.3 | 4.1 ± 0.9 |
| V ss (mL/kg) | 68.3 ± 10.4 | 66.1 ± 16.0 |
AUC = Area under the curve (Factor VIII:C); AUC norm = AUC divided by the dose; C maxnorm = Maximal plasma concentration divided by the dose; CL = Clearance;Factor VIII:C = Factor VIII coagulation activity; IVR = Incremental in vivo recovery; MRT = Mean residence time; PK = Pharmacokinetics; SD = Standard deviation; T 1/2 = Terminal half-life; V ss = Volume of distribution at steady state; * Median, lower/upper quartile: 10.1, 9.4/13.7; # Median, lower/upper quartile: 12.8, 11.2/15.9
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
The efficacy of NUWIQ was evaluated in three multi-center, open-label, prospective clinical trials in PTPs with severe Hemophilia A. For routine prophylaxis, the efficacy of NUWIQ was evaluated in two multi-center studies, one in adult patients (n = 32) and one in pediatric patients (n = 59). For the treatment of bleeding episodes, efficacy was evaluated in one multi-center study in adolescents (n = 2) and adults (n = 20) who were treated on-demand only, and also in patients who experienced breakthrough bleeding episodes in the two prophylaxis studies. Across all studies, subjects undergoing surgical procedures were evaluated for hemostatic efficacy during perioperative management.
On-demand Treatment and Control of Bleeding Episodes
A total of 1124 bleeding episodes in 69 subjects (35 adults, 2 adolescents, and 32 children) were treated with NUWIQ. Response to each treatment was assessed by the patients using an ordinal scale of excellent (abrupt pain relief and/or unequivocal improvement in objective signs of bleeding within approximately 8 hours after a single infusion), good (definite pain relief and/or improvement in signs of bleeding within approximately 8–12 hours after an infusion requiring up to 2 infusions for complete resolution), moderate (probable or slight beneficial effect within approximately 12 hours after the first infusion requiring more than two infusions for complete resolution), or none (no improvement within 12 hours, or worsening of symptoms, requiring more than 2 infusions for complete resolution).
The majority of treated bleeding episodes (n = 986) was from the study where patients only received on-demand treatment. 642 (65%) bleeding episodes occurred spontaneously, 341 (35%) were traumatic, and 3 (0.3%) bleeding episodes were due to other causes. The mean dose per injection used to treat a bleeding episode was 32 IU/kg. Hemostatic efficacy in response to NUWIQ treatment was rated as excellent or good in 94% and as moderate in 6% of the bleeds.
In case of breakthrough bleeding episodes, the mean dose per injection used to treat a bleeding episode was 33.3 IU/kg in adults (n=15 with 30 bleeding episodes) and 45 IU/kg in pediatric patients (n=32 with 108 bleeding episodes). The median number of injections to treat a bleeding episode was 1. Hemostatic efficacy was excellent or good in 100% of bleeds in adults and 82% of bleeds in pediatric patients.
Perioperative Management of Bleeding
Across all studies, the efficacy of NUWIQ in surgical prophylaxis was assessed in a total of 60 surgical procedures in 36 patients; 32 procedures in 16 patients were classed as minor and 28 procedures in 23 patients were classed as major. NUWIQ pre-operative dosing ranged from 33 IU/kg to 90 IU/kg per infusion. The total number of infusions administered ranged from one to 19 for minor procedures and three to 76 for major procedures; three procedures required an injection of NUWIQ during surgery.
The efficacy of surgical prophylaxis was rated for each case by a surgeon and a hematologist, taking into account both the intra- and postoperative assessment. Hemostasis efficacy was rated at the end of the surgery by the surgeon and postoperatively by the surgeon and hematologist using ordinal scales as follows:
- Excellent: Intra-operative: intra-operative blood loss lower than or equal to the average expected blood loss for the type of procedure performed in a patient with normal hemostasis; Postoperative: No postoperative bleeding or oozing that was not due to complications of surgery. All postoperative bleeding (due to complications of surgery) was controlled with NUWIQ as anticipated for the type of procedure.
- Good: Intra-operative: intra-operative blood loss was higher than average expected blood loss but lower than or equal to the maximal expected blood loss for the type of procedure in a patient with normal hemostasis; Postoperative: No postoperative bleeding or oozing that was not due to complications of surgery. Control of postoperative bleeding due to complications of surgery required increased dosing with NUWIQ or additional infusions, not originally anticipated for the type of procedure.
- Moderate: Intra-operative: Intra-operative blood loss was higher than maximal expected blood loss for the type of procedure performed in a patient with normal hemostasis, but hemostasis was controlled. Postoperative: Some postoperative bleeding and oozing that was not due to complications of surgery; control of postoperative bleeding required increased dosing with NUWIQ or additional infusions, not originally anticipated for the type of procedure.
- None: Intra-operative: Hemostasis was uncontrolled necessitating a change in clotting factor replacement regimen. Postoperative: Extensive uncontrolled postoperative bleeding and oozing. Control of postoperative bleeding required use of an alternate FVIII concentrate.
Efficacy for 28 major surgeries was rated as excellent in 23 (82%) cases, good in 4 (14%) cases, and moderate in 1 (4%) case. The efficacy of all 30 rated minor surgeries was excellent.
Routine Prophylaxis and Bleeding Control
In the study evaluating the efficacy and safety of NUWIQ for routine prophylaxis in 32 adult subjects (29 White, 3 Asian), the product was given every other day with a dose of 30-40 IU/kg for at least 6 months. In another study evaluating the safety, immunogenicity and hemostatic efficacy in 59 pediatric subjects aged 2 to 12 years (all White, 29 were 2 to 5 years old, and 30 between 6 and 12 years), subjects received NUWIQ prophylactically every other day or 3 times per week for at least 6 months. Clinical outcomes are summarized in Table 6 .
Table 6. Clinical Outcomes in Adult and Pediatric Subjects
| Adults (N=32) | Children (N=59) | |
| Mean dose (± standard deviation) | 32.8 ± 2.8 IU/kg | 38.9 ± 7.2 IU/kg |
| Subjects with 0 bleeding episodes | 16 (50.0%) | 20 (33.9%) |
| Subjects with 1 bleeding episode | 11 (34.4%) | 14 (23.7%) |
| Subjects with 2 bleeding episodes | - | 3 (5.1%) |
| Subjects with ≥ 3 bleeding episodes | - | 22 (37.3%) |
| Subjects with ≥5 bleeding episodes | 5 (15.6%) | |
| Annualized bleeding rate (per subject) - spontaneous bleeds | 1.16 ± 2.57 (median 0, range 0-8.6) | 1.50 ± 3.32 (median 0, range 0-13.8) |
| Annualized bleeding rate (per subject) for all types of bleeds | 2.28 ± 3.73 (median 0.9, range 0-14.7) | 4.12 ± 5.22 (median 1.90, range 0-20.7) |
| Reduction in annualized bleeding rate compared to on-demand treatment in a different study* | 96% | 93% |
Severity of bleeds (% of bleeds) in the adults were major 16 (36.4%), minor – 28 (63.6%), life threatening 0. Severity of bleeds in the children was moderate or major 64 (42.6%), minor 61 (56.5%), unknown 1 (0.9%), life threatening 0. * Based on a negative binomial model.
In a study of 59 previously treated pediatric patients ages 2-12 years, the children received a total of 5746 infusions. Of these infusions, 5316 (93%) were for prophylaxis, 216 (4%) for the treatment of bleeding episodes, 41 (0.7%) for peri-operative management and 173 (3%) for pharmacokinetic (PK) and recovery assessments.
Long-term treatment with routine prophylaxis was evaluated in an extension of the pediatric study in which 49 children (2-5 years [N=26] and 6-12 years [N=23]) who had completed the original pediatric study were treated with an additional 20,518 infusions of NUWIQ over a mean of an additional 29.4 months. Across both studies, a total of 26,289 infusions and 33,724,769 IU (990,927 IU/kg) were given. Of these infusions, 25,040 (95.2%) were for prophylaxis, 700 (2.7%) for treatment of bleeding episodes, 304 (1.2%) for peri-operative management, 247 (0.9%) for recovery assessments, and 189 (0.7%) for pharmacokinetic analyses. The median dose per prophylactic infusion was 37 IU/kg (range 12.8-124 IU/kg).
The mean annualized bleeding rate was 3.5 ± 4.4 (median 2.2, range 0.0 – 24.7). Eight of the 59 children (14%) had no bleeds.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- NUWIQ is supplied in packages comprising a single-use vial containing nominally 250, 500, 1000 2000, 2500, 3000 or 4000 international units (IU) of Factor VIII potency, a pre-filled syringe with 2.5 mL solvent (Water for Injection), a vial adapter, a butterfly needle and two alcohol swabs. The actual amount of NUWIQ in IU is stated on each carton and vial.
- Components used in the packaging of NUWIQ are not made with natural rubber latex.
| Container NDC | Carton NDC | ||
| pale blue | NUWIQ 250 IU | 68982-140-01 | 68982-139-01 |
| pale pink | NUWIQ 500 IU | 68982-142-01 | 68982-141-01 |
| green blue | NUWIQ 1000 IU | 68982-144-01 | 68982-143-01 |
| purple | NUWIQ 1500 IU | 68982-154-01 | 68982-153-01 |
| orange | NUWIQ 2000 IU | 68982-146-01 | 68982-145-01 |
| brown | NUWIQ 2500 IU | 68982-148-01 | 68982-147-01 |
| dark grey | NUWIQ 3000 IU | 68982-150-01 | 68982-149-01 |
| dark green | NUWIQ 4000 IU | 68982-152-01 | 68982-151-01 |
Storage and Handling
- Store NUWIQ in the original package to protect the NUWIQ vials from light.
- Store NUWIQ in powder form at 2 – 8°C (35 – 46°F) for up to 24 months. Do not freeze.
- During the shelf life, the product may be kept at room temperature [up to 25°C (77°F)] for a single period not exceeding 3 months. After storage at room temperature, do not return the product to the refrigerator.
- Do not use after the expiration date.
- Keep the reconstituted solution at room temperature. Do not refrigerate after reconstitution. Use the reconstituted solution immediately or within 3 hours after reconstitution. Discard any remaining solution.
17 PATIENT COUNSELING INFORMATION
- Advise patients to read the FDA-approved patient labeling ( Patient Information and Instructions for Use )
- Because hypersensitivity reactions are possible with NUWIQ , inform patients of the early signs of hypersensitivity reactions, including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. Advise patients to stop the injection if any of these symptoms arise and contact their physician, and seek prompt emergency treatment.
- Advise patients to contact their physician or treatment center for further treatment and/or assessment if they experience a lack of clinical response to Factor VIII replacement therapy, as this may be a manifestation of an inhibitor.
- Advise patients to consult with their healthcare provider prior to traveling. While traveling, patients should be advised to bring an adequate supply of NUWIQ based on their current treatment regimen.
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
Distributed by:
Octapharma USA, Inc.
117 West Century Road
Paramus, NJ 07652
PATIENT PACKAGE INSERT
FDA-APPROVED PATIENT LABELING
Patient Information
NUWIQ /nu’ veek /
Antihemophilic Factor (Recombinant)
Please read this Patient Information carefully before using NUWIQ and each time you get a refill, as there may be new information. This Patient Information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is NUWIQ ?
NUWIQ is an injectable medicine that is used to help treat and control bleeding in adults and children with Hemophilia A (congenital clotting Factor VIII deficiency). NUWIQ can reduce the number of bleeding episodes in children and adults when used regularly (prophylaxis). Usually, Hemophilia A treatment is life-long.
Your healthcare provider may also give you NUWIQ when you have surgery.
NUWIQ is NOT used to treat von Willebrand disease.
Who should not use NUWIQ ?
You should not use NUWIQ if you had an allergic reaction to it in the past.
Tell your healthcare provider if you are (or are planning to become) pregnant and/or breastfeeding because NUWIQ may not be right for you.
What should I tell my healthcare provider before using NUWIQ ?
Talk to your healthcare provider about any medical conditions that you have or have had, including if you have been told that you have inhibitors to Factor VIII, because NUWIQ may not work for you.
Tell your healthcare provider about all of the prescription and non-prescription medicines you take, including over-the-counter medicines, dietary supplements, and/or herbal medicines.
How should I use NUWIQ ?
You get NUWIQ as an infusion into your vein. NUWIQ is sold as a powder in a vial. The powder is mixed with sterile water supplied in a prefilled syringe. See instructions for reconstitution and injection of NUWIQ.
Your healthcare provider will instruct you on how to do reconstitutions and infusions on your own or with the help of a family member. Your healthcare provider may watch you give yourself the first dose of NUWIQ .
You must carefully follow your healthcare provider’s instructions regarding the dose and schedule for infusing NUWIQ so that your treatment will work optimally for you.
NUWIQ comes in different dosage strengths. The actual number of international units (IU) of Factor VIII in the vial will be printed on the label and box. Always check the actual number of IU of Factor VIII printed on the label to make sure you are using the strength prescribed by your healthcare provider..
Contact your healthcare provider right away if bleeding is not controlled after using NUWIQ .
Talk to your healthcare provider before travelling. Plan to bring enough NUWIQ for your treatment during this time.
Do not stop using NUWIQ without consulting with your healthcare provider.
What are the possible side-effects of NUWIQ ?
Allergic reactions may occur with NUWIQ. Stop the injection immediately and call your healthcare provider or emergency department right away if you have any of the following symptoms: dizziness, loss of consciousness, difficulty breathing, wheezing, chest tightness, swelling of lips and tongue, rash, or hives.
Your body can also make antibodies (known as inhibitors) against Factor VIII, which may stop NUWIQ from working properly. Your healthcare provider may test your blood to check for inhibitors at regular intervals.
Side-effects that have been reported with NUWIQ include: injection site inflammation, injection site pain, prickling or tingling sensation, headache, back pain, dizziness, and dry mouth.
These are not all the possible side effects of NUWIQ. Talk to your healthcare provider about any side-effect that bothers you or that does not go away.
How should I store NUWIQ ?
Keep NUWIQ in its original box to protect it from exposure to light. Do not freeze NUWIQ .
You can store NUWIQ in the refrigerator for up to 24 months at 2-8°C (36-46°F). NUWIQ can be kept at room temperature [up to 25°C (77°F)] for a single period not exceeding 3 months (note on the carton the date when the product was removed from the refrigerator). After storage at room temperature, the product must be used or discarded, and it must not be put back into the refrigerator.
Do not use NUWIQ after the expiration date printed on the vial.
Do not use NUWIQ if the reconstituted solution is cloudy, contains particles, and/or is not colorless.
NUWIQ should be used as soon as possible after reconstitution. Protect reconstituted NUWIQ from light and temperatures above 25°C (77°F). Discard any product not used within three hours.
Dispose of all materials, including any unused NUWIQ , in an appropriate container.
What else should I know about NUWIQ ?
Do not use NUWIQ for a medical condition for which it was not prescribed. Do not share NUWIQ with other people, even if they have the same diagnosis and symptoms that you have.
Resources at Octapharma available to patients
For more product information on NUWIQ , please visit www.NUWIQ.com.
For more information on patient assistance programs that are available to you, please contact the Octapharma Patient Support Center at 1-800-554-4440.
For more information on additional Octapharma patient resources, please visit www.NUWIQ.com.
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
Distributed by:
Octapharma USA, Inc.
117 West Century Road
Paramus, NJ 07652
NUWIQ is a registered trademark of Octapharma.
INSTRUCTIONS FOR USE
NUWIQ /nu’ veek /
Antihemophilic Factor (Recombinant)
Read these instructions carefully before using NUWIQ for the first time. You should ensure that you have the appropriate training from your healthcare provider or hemophilia treatment center before attempting a self-infusion of NUWIQ. Always follow the prescribed dose and specific instructions given by your healthcare provider. The general guidelines for mixing and infusing NUWIQ are listed below. If you are unsure of any of these steps, please contact your healthcare provider before using NUWIQ.
Instruction for Mixing NUWIQ
- Always work on a clean surface and wash your hands before performing the procedure.
- Allow the vial of NUWIQ and the pre-filled syringe to come to room temperature.
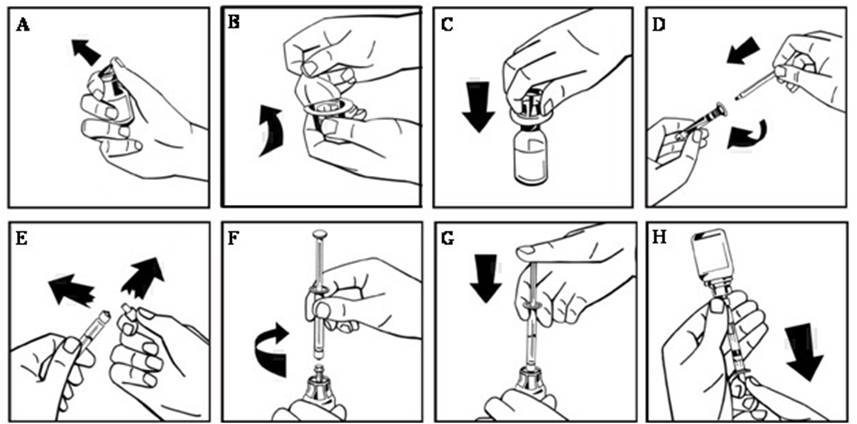
- Remove the plastic flip-top cap from the NUWIQ vial to expose the rubber stopper (Figure A).
- Wipe the top of the vial with an alcohol swab and allow the rubber stopper of the vial to dry.
- Peel back the paper cover from the vial adapter package revealing the adapter spike without removing the adapter from the package (Figure B).
- With the vial on an even surface, insert the adapter spike into the rubber stopper. The adapter snaps to the vial when done (Figure C).
- Peel back the paper cover from the pre-filled syringe package. Connect plunger rod attaching the threaded end of the plunger rod to the solvent syringe, turning clockwise until a slight resistance is felt (Figure D). Avoid contact with the shaft of the plunger rod.
- Break off the tamper-proof plastic tip from the syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip (Figure E).
- Remove the adapter packaging and connect the syringe to the vial adapter by turning clockwise until resistance is felt (Figure F).
- Slowly inject all liquid from syringe into the concentrate vial (Figure G).
- Without removing the syringe, dissolve the concentrate powder in the vial by gently moving or swirling a few times. DO NOT SHAKE. Wait until all the powder dissolves completely.
- Inspect the final solution for particles. The solution should be clear, colorless, and free from visible particles. Do not use if solution is cloudy or if it has visible particles.
- Turn the vial and syringe upside down (still attached).
- Slowly withdraw the solution into the syringe. Make sure that all liquid is transferred to the syringe (Figure H).
- Detach the filled syringe from the vial adapter by turning counter clockwise.
- Do not refrigerate the solution after reconstitution. Use the solution within 3 hours after reconstitution. If solution is not used within this time period, close the filled syringe with the tamper-proof plastic tip and discard the syringe.
Instructions for Injecting NUWIQ
For intravenous use after reconstitution only.
- Inspect the reconstituted NUWIQ solution for visible particulate matter and discoloration prior to administration. Do not use if particles and/or discoloration are observed.
- Do not administer NUWIQ in the same tubing or container as other medications.
- Clean the chosen injection site with an alcohol swab.
- Attach the provided infusion set to the syringe. Insert the needle of the infusion set into the chosen vein.
- Perform intravenous bolus infusion. The rate of administration should be determined by the patient’s comfort level, at a maximum rate of 4 mL per minute.
- After infusing NUWIQ, remove and properly discard the infusion set. After the infusion, remove the peel-off label containing the batch number from the factor concentrate vial and place it in the log book for record keeping. Discard the empty vial.
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
Distributed by:
Octapharma USA, Inc.
117 West Century Road
Paramus, NJ 07652
NUWIQ is a registered trademark of Octapharma.
Issued September 2020.
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
Vial Label – Principal Display Panel
250 IU Range
NDC 68982-140-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646

Carton Label – Principal Display Panel
250 IU Range
NDC 68982-139-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
Vial Label – Principal Display Panel
500 IU Range
NDC 68982-142-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646

Carton Label – Principal Display Panel
500 IU Range
NDC 68982-141-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
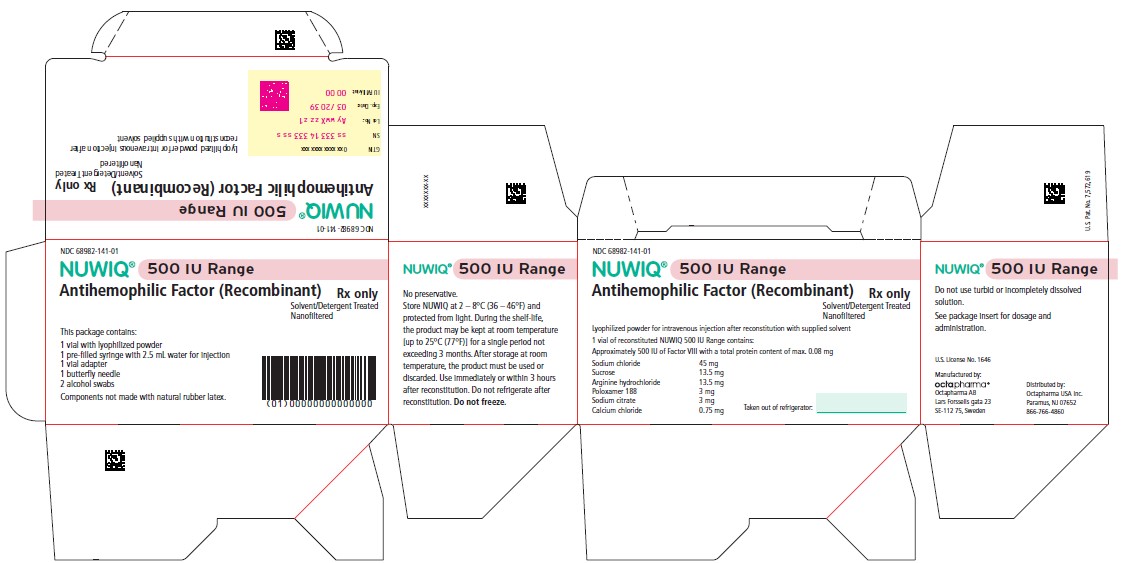
Vial Label – Principal Display Panel
1000 IU Range
NDC 68982-144-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646

Carton Label – Principal Display Panel
1000 IU Range
NDC 68982-143-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
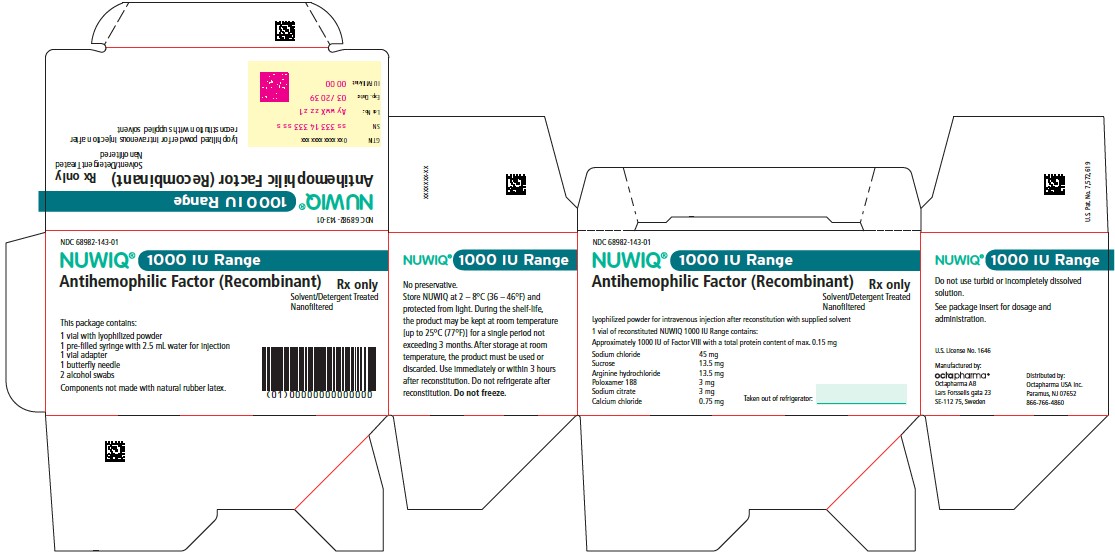
Vial Label – Principal Display Panel
1500 IU Range
NDC 68982-154-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646

Carton Label – Principal Display Panel
1500 IU Range
NDC 68982-153-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
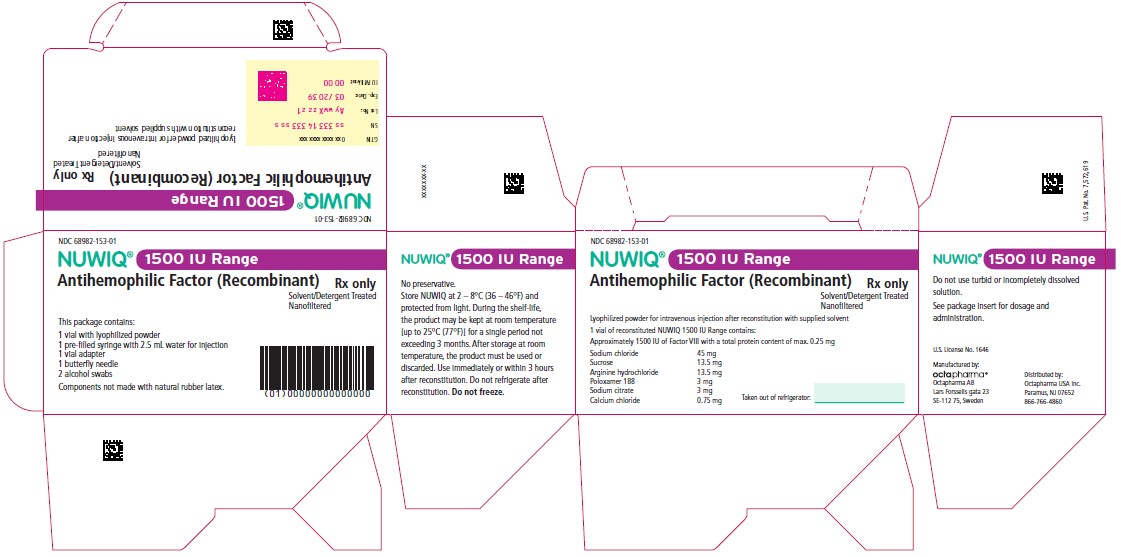
Vial Label – Principal Display Panel
2000 IU Range
NDC 68982-146-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646

Carton Label – Principal Display Panel
2000 IU Range
NDC 68982-145-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
Vial Label – Principal Display Panel
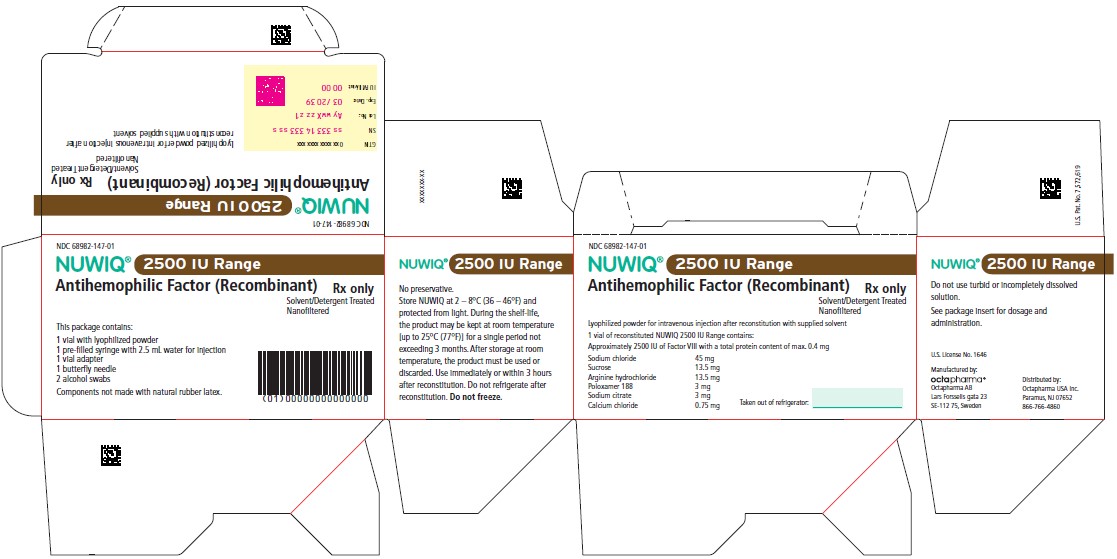
2500 IU Range
NDC 68982-148-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646

Carton Label – Principal Display Panel
2500 IU Range
NDC 68982-147-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
Vial Label – Principal Display Panel
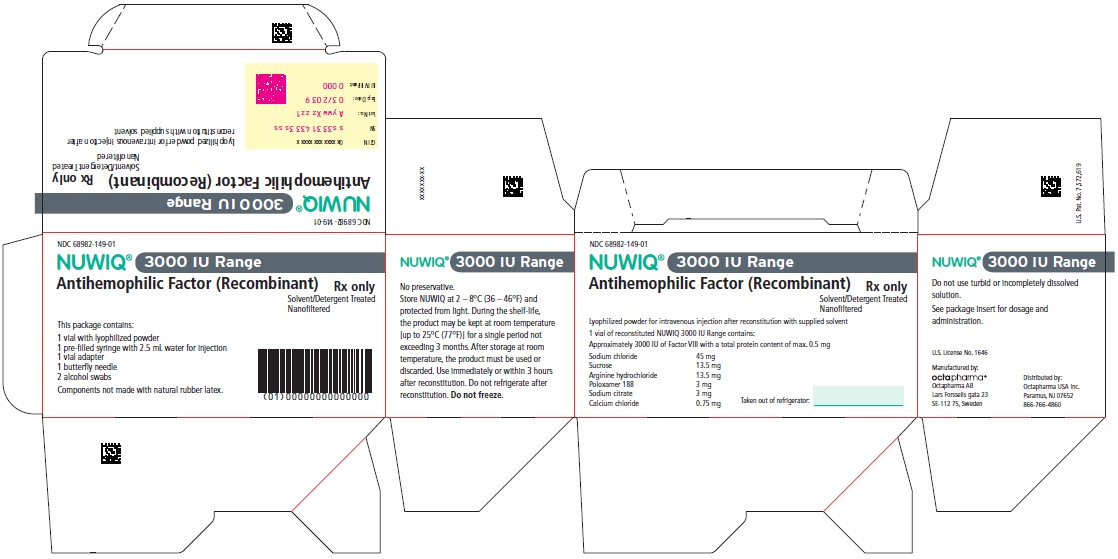
3000 IU Range
NDC 68982-150-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646

Carton Label – Principal Display Panel
3000 IU Range
NDC 68982-149-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
Vial Label – Principal Display Panel
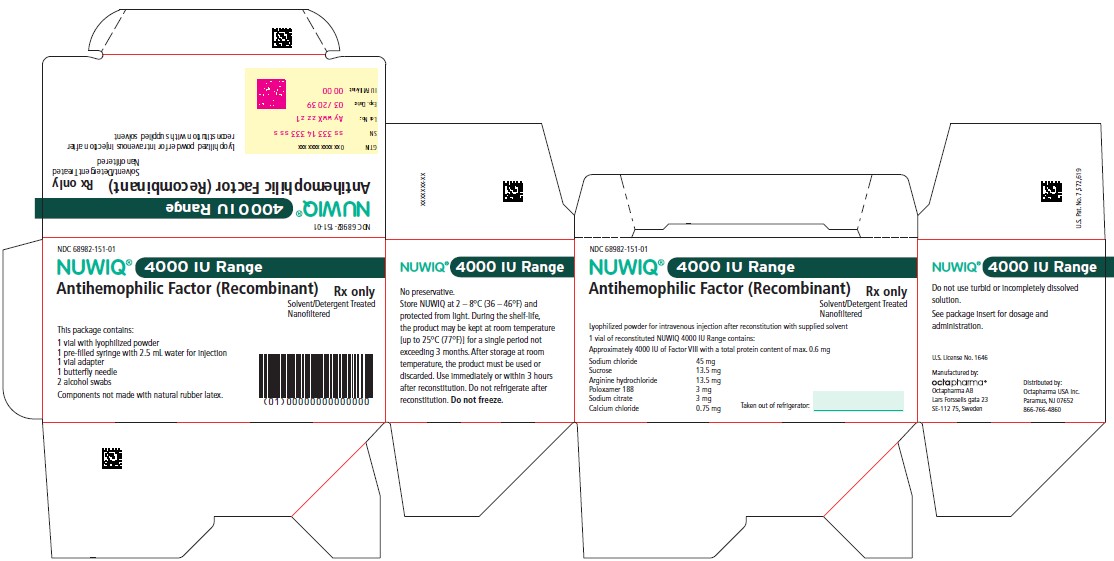
4000 IU Range
NDC 68982-152-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646

Carton Label – Principal Display Panel
4000 IU Range
NDC 68982-151-01
NUWIQ ®
Antihemophilic Factor (Recombinant)
Lyophilized Powder for Solution for Intravenous Injection
Manufactured by:
Octapharma AB
Lars Forssells gata 23
SE-112 75, Sweden
U.S. License No. 1646
Vial Label – Principal Display Panel
2.5 mL Water for Injection for reconstitution of NUWIQ
