Use
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 1 to 3 days
Warnings
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
Directions (Bottle Only)
- do not take more than directed unless advised by your doctor
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- adults and children 17 years of age and older:
- use once a day
- fill to top of white section in cap which is marked to indicate the correct dose (17 g)
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickeners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use no more than 7 days
- children 16 years of age or under: ask a doctor
Directions (Packet Only)
- do not take more than directed unless advised by your doctor
- adults and children 17 years of age and older:
- use once a day
- stir and dissolve one packet of powder (17 g) in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickeners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use more than 7 days
- children 16 years of age or under: ask a doctor
Other information
- store at 20°-25°C (68°-77°F)
- tamper-evident: do not use if foil seal under cap, printed with "SEALED for YOUR PROTECTION" is missing, open or broken
PRINCIPAL DISPLAY PANEL - 17 g × 20 Packet Carton
20
Single Doses
#1 DOCTOR RECOMMENDED BRAND
MiraLAX ®
Polyethylene Glycol 3350, Powder for Solution, Osmotic Laxative
Mix-In Pax ®
- Relieves Occasional Constipation/Irregularity
- Softens Stool
20
ONCE-DAILY DOSES
Bayer
Unflavored Powder Dissolves in ANY Beverage!
UNFLAVORED
POWDER
GRIT FREE
20 PACKETS
NET WT 0.5 OZ
(17g) EACH

PRINCIPAL DISPLAY PANEL - 17 g × 10 Packet Carton
10
Single Doses
#1 DOCTOR RECOMMENDED BRAND
MiraLAX
®
Polyethylene Glycol 3350, Powder for Solution, Osmotic Laxative
Mix-In Pax ®
- Relieves Occasional Constipation/Irregularity
- Softens Stool
10
ONCE-DAILY DOSES
Bayer
Unflavored Powder Dissolves in ANY Beverage!
UNFLAVORED
POWDER
GRIT FREE
10 PACKETS
NET WT 0.5 OZ
(17g) EACH

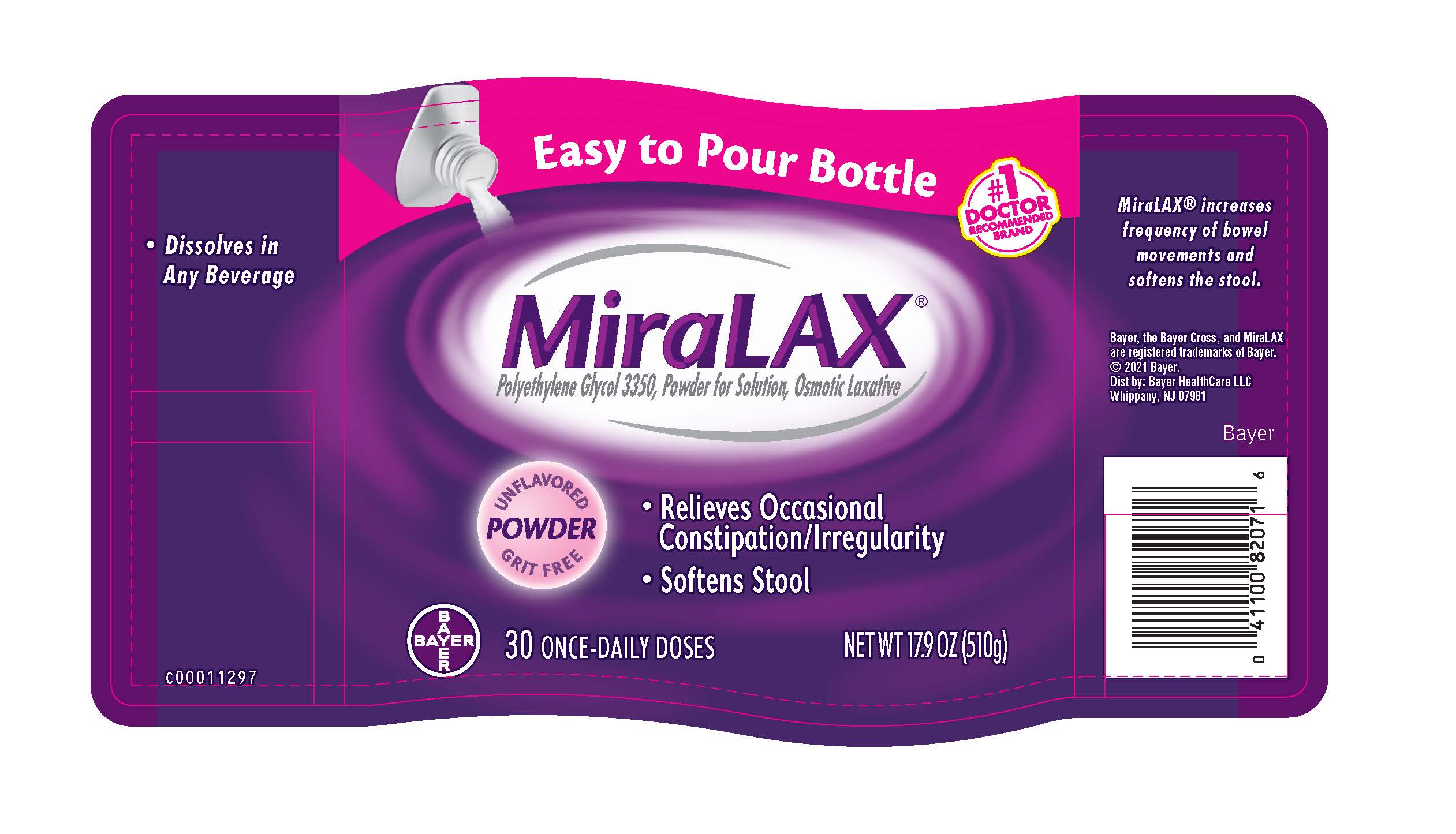
PRINCIPAL DISPLAY PANEL - 30 Dose (510 g) Bottle Label
Easy to Pour Bottle
#1 DOCTOR RECOMMENDED BRAND
MiraLAX ®
Polyethylene Glycol 3350, Powder for Solution, Osmotic Laxative
UNFLAVORED
POWDER
GRIT FREE
- Relieves Occasional Constipation/Irregularity
- Softens Stool
Bayer
30 ONCE-DAILY DOSES
NET WT 17.9 OZ (510 g)

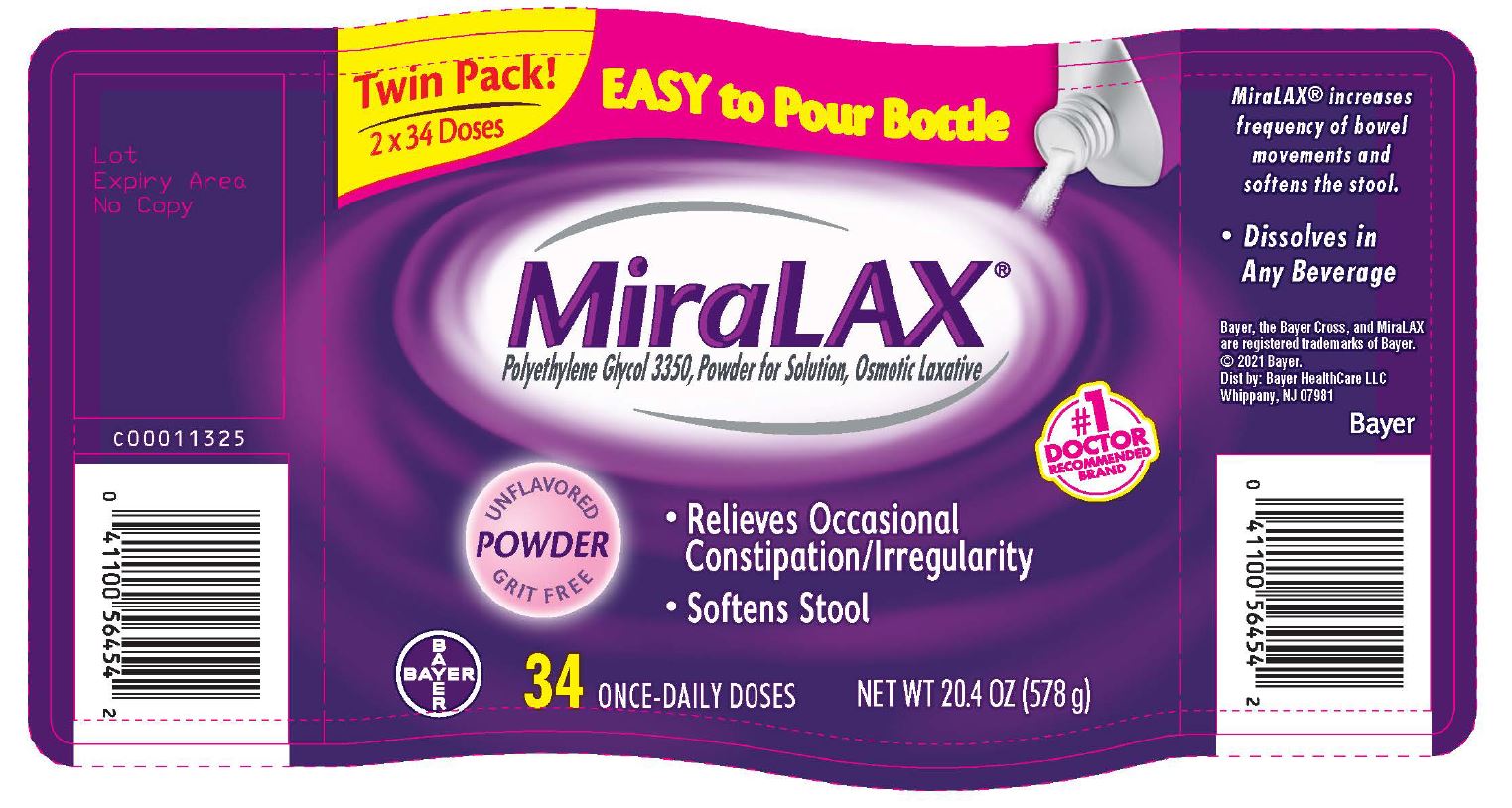
PRINCIPAL DISPLAY PANEL - Twinpack 2x34 Dose (578 g) Bottle Label
Twin Pack!
2 x 34 Doses
Easy to Pour Bottle
MiraLAX ®
Polyethylene Glycol 3350, Powder for Solution, Osmotic Laxative
#1 DOCTOR RECOMMENDED BRAND
- Relieves Occasional Constipation/Irregularity
- Softens Stool
UNFLAVORED
POWDER
GRIT FREE
Bayer
34 ONCE-DAILY DOSES
NET WT 20.4 OZ (578 g)