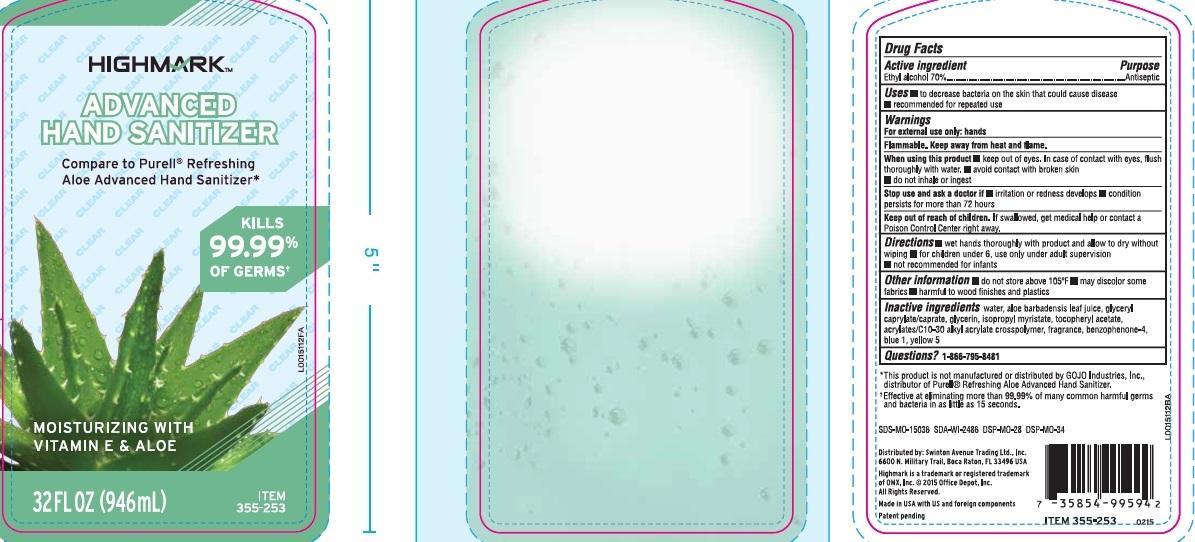
When using this product
- keep out of eyes. In case of contact with eyes, flush thoroughly with water
- avoid contact with broken skin
- do not inhale or ingest
Stop use and ask a doctor if
- irritation or redness develops
- condition persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wet hands thoroughly with product and allow to dry without wiping
- for children under 6, use only under adult supervision
- not recommended for infants
Other information
- do not store above 105⁰ F
- may discolor some fabrics
- harmful to wood finishes and plastics
Inactive ingredients
water, aloe barbadensis leaf juice, glyceryl caprylate/caprate, glycerin, isopropyl myristate, tocopheryl acetate, acrylates/C10-30 alkyl acrylate crosspolymer, fragrance, benzophenone-4, blue 1, yellow 5
*This product is not manufactured or distributed by GOJO Indutries, Inc., distributor of Purell Refreshing Aloe Advanced Hand Sanitizer
**Effective at eliminating more than 99.99% of many common harmful germs and bacteria in as little as 15 seconds.
Distributed by: Switon Avenue Trading Ltd., Inc.
6600 N. Military Trail, Boca Raton, FL 33496
Highark is a trademark or registered trademark of OMX, Inc. Office Depot, Inc
Made in USA with US and foreign components
SDS-MO-15036 SDA-WI-2486 DSP-MO-28 DSP-MO-34