ADVANCED HAND SANITIZER- hand sanitizer liquid
Dongguang Shuoguo Silicone Products Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
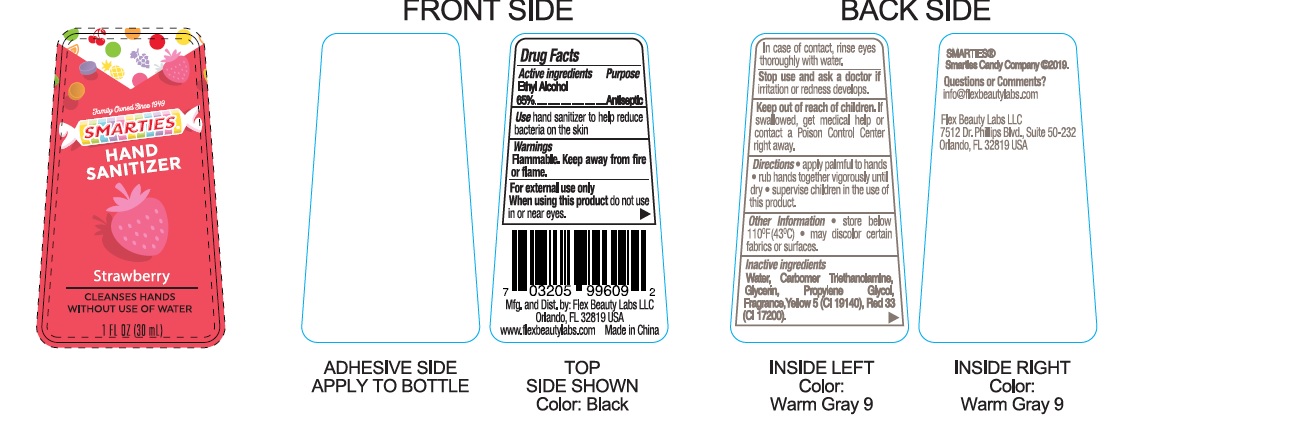
Drug Facts
Active Ingredient
Ethyl Alcohol 65.0%
Uses
Hand Santizer to help reduce bacteria on skin that may cause disease.
Warnings
Flammable. Keep away from heat or flame.
For external use only.
Keep out of reach of children.
When using this product,
do not use in or near the eyes. In case of contact, rinse eyes thoroughtly with water. Stop use and ask doctor if irritation or rash appears and lasts. Keep out of reach for children.
If swallowed, get medical help or contact a Posion Control Center right away.
Directions:
. Place enough Product in your palm to thoroughtly spread on both hands and rub into the skin until dry.
. Children under 6 years of age should be supervised when using this product.
Other Information:
. Store below 106℉. (41℃)
Inactive Ingredients:
Water (Aqua), Carbomer triethanolamine, Fragrance Red 33, Glycerin, Propylene Glycol, Yellow 5
Dongguang Shuoguo Silicone Products Co., Ltd.