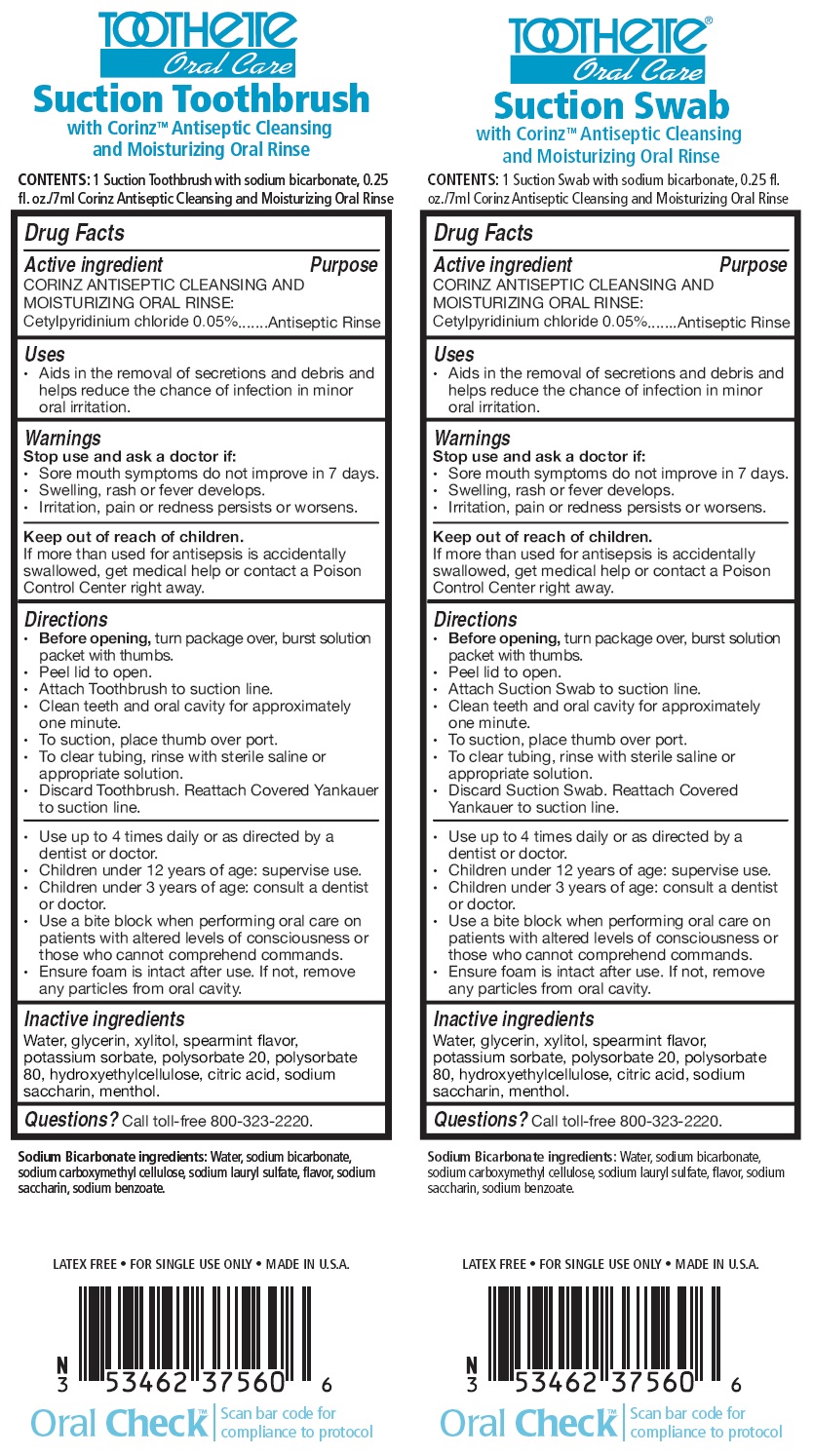
Drug Facts
| Active ingredient: | Purpose |
| CORINZ ANTISEPTIC CLEANSING AND MOISTURIZING ORAL RINSE: | |
| Cetylpyridinium chloride 0.05% | Antiseptic Rinse |
Uses
- Aids in the removal of secretions and debris and helps reduce the chance of infection in minor oral irritation.
Warnings
Stop use and ask a doctor if:
- Sore mouth symptoms do not improve in 7 days.
- Swelling, rash or fever develops.
- Irritation, pain or redness persists or worsens.
Keep out of
reach of children.
If more than used for antisepsis
is accidentally swallowed, get medical help or contact a Poison Control
Center right away.
Directions
Toothette Oral Care Suction Toothbrush
- Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Attach Toothbrush to suction line.
- Clean teeth and oral cavity for approximately one minute.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Toothbrush. Reattach Covered Yankauer to suction line.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Toothette Oral Care Suction Swab
- Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Attach Suction Swab to suction line.
- Clean teeth and oral cavity for approximately one minute.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Swab. Reattach Covered Yankauer to suction line.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.