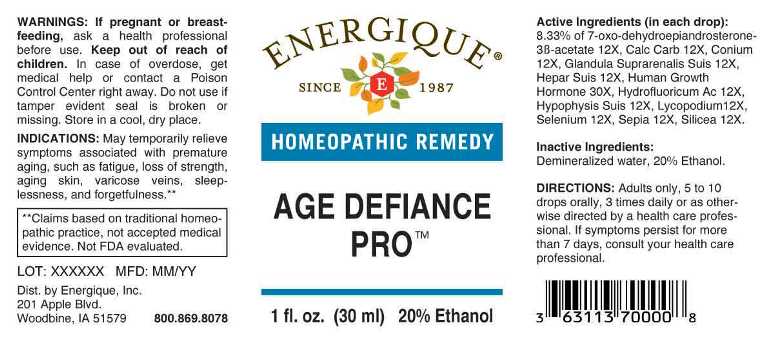
ACTIVE INGREDIENTS:
(in each drop): 8.33% of 7-Oxo-Dehydroepiandrosterone-3B-Acetate 12X, Calcarea Carbonica 12X, Conium Maculatum 12X, Glandula Suprarenalis Suis 12X, Hepar Suis 12X, Human Growth Hormone 30X, Hydrofluoricum Acidum 12X, Hypophysis Suis 12X, Lycopodium Clavatum 12X, Selenium Metallicum 12X, Sepia 12X, Silicea 12X.
INDICATIONS:
May temporarily relieve symptoms associated with premature aging, such as fatigue, loss of strength, aging skin, varicose veins, sleeplessness and, forgetfulness.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults only, 5 to 10 drops orally, 3 times daily or as otherwise directed by a health professional. If symptoms persist for more than 7 days, consult your health care professional.