DESCRIPTION
Naftifine Hydrochloride Cream USP, 1% contains the synthetic, broad-spectrum, antifungal agent naftifine hydrochloride. Naftifine Hydrochloride Cream USP, 1% is for topical use only.
CHEMICAL NAME:
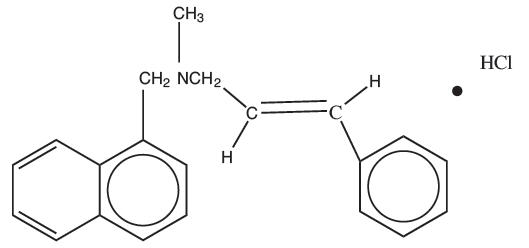
(E)-N-Cinnamyl-N-methyl-1-naphthalenemethylamine hydrochloride. Naftifine hydrochloride has an empirical formula of C21H21N∙HCl and a molecular weight of 323.86.
Structural Formula
|
|
| naftifine hydrochloride |
Contains
Active Ingredient
Naftifine hydrochloride 1%
Inactive Ingredients
benzyl alcohol, cetyl alcohol, cetyl esters wax, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, and stearyl alcohol. Hydrochloric acid may be added to adjust pH.
CLINICAL PHARMACOLOGY
Naftifine hydrochloride is a synthetic allylamine derivative. The following in vitro data are available, but their clinical significance is unknown. Naftifine hydrochloride has been shown to exhibit fungicidal activity in vitro against a broad spectrum of organisms, including Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Epidermophyton floccosum, Microsporum canis, Microsporum audouini, and Microsporum gypseum; and fungistatic activity against Candida species, including Candida albicans. Naftifine Hydrochloride Cream USP, 1% has only been shown to be clinically effective against the disease entities listed in the INDICATIONS AND USAGE section.
Although the exact mechanism of action against fungi is not known, naftifine hydrochloride appears to interfere with sterol biosynthesis by inhibiting the enzyme squalene 2, 3-epoxidase. This inhibition of enzyme activity results in decreased amounts of sterols, especially ergosterol, and a corresponding accumulation of squalene in the cells.
Pharmacokinetics
In vitro and in vivo bioavailability studies have demonstrated that naftifine penetrates the stratum corneum in sufficient concentration to inhibit the growth of dermatophytes.
Following a single topical application of 1% naftifine cream to the skin of healthy subjects, systemic absorption of naftifine was approximately 6% of the applied dose. Naftifine and/or its metabolites are excreted via the urine and feces with a half-life of approximately two to three days.
INDICATIONS AND USAGE
Naftifine Hydrochloride Cream USP, 1% is indicated for the topical treatment of tinea pedis, tinea cruris and tinea corporis caused by the organisms Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum.
CONTRAINDICATIONS
Naftifine Hydrochloride Cream USP, 1% is contraindicated in individuals who have shown hypersensitivity to any of its components.
PRECAUTIONS
General
Naftifine Hydrochloride Cream USP, 1% is for external use only. If irritation or sensitivity develops with the use of Naftifine Hydrochloride Cream USP, 1%, treatment should be discontinued and appropriate therapy instituted.
Diagnosis of the disease should be confirmed either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide or by culture on an appropriate medium.
Information for patients
The patient should be told to:
- Avoid the use of occlusive dressings or wrappings unless otherwise directed by the physician.
- Keep Naftifine Hydrochloride Cream USP, 1% away from the eyes, nose, mouth and other mucous membranes.
Carcinogenesis, mutagenesis, impairment of fertility
In a 2-year dermal carcinogenicity study, naftifine hydrochloride cream was administered to Sprague-Dawley rats at topical doses of 1%, 2% and 3% (10 mg/kg/day, 20 mg/kg/day, and 30 mg/kg/day naftifine hydrochloride). No drug-related tumors were noted in this study up to the highest dose evaluated in this study of 30 mg/kg/day [3.6 times the maximum recommended human dose (MRHD) based on mg/m2 comparison].
Naftifine hydrochloride revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and Chinese hamster ovary cell chromosome aberration assay) and one in vivo genotoxicity test (mouse bone marrow micronucleus assay).
Oral administration of naftifine hydrochloride to rats, throughout mating, gestation, parturition and lactation, demonstrated no effects on growth, fertility or reproduction, at doses up to 100 mg/kg/day (12 times MRHD based on mg/m2 comparison).
Pregnancy
Teratogenic Effects
Reproduction studies have been performed in rats and rabbits (via oral administration) at doses 150 times or more than the topical human dose and have revealed no evidence of impaired fertility or harm to the fetus due to naftifine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
ADVERSE REACTIONS
During clinical trials with Naftifine Hydrochloride Cream USP, 1%, the incidence of adverse reactions was as follows: burning/stinging (6%), dryness (3%), erythema (2%), itching (2%), local irritation (2%).
DOSAGE AND ADMINISTRATION
A sufficient quantity of Naftifine Hydrochloride Cream USP, 1% should be gently massaged into the affected and surrounding skin areas once a day. The hands should be washed after application. If no clinical improvement is seen after four weeks of treatment with Naftifine Hydrochloride Cream USP, 1%, the patient should be re-evaluated.
HOW SUPPLIED
Naftifine Hydrochloride Cream USP, 1% is supplied in the following sizes:
15 g - NDC 51672-1362-1 (tube)
30 g - NDC 51672-1362-2 (tube)
60 g - NDC 51672-1362-3 (tube)
90 g - NDC 51672-1362-8 (tube)