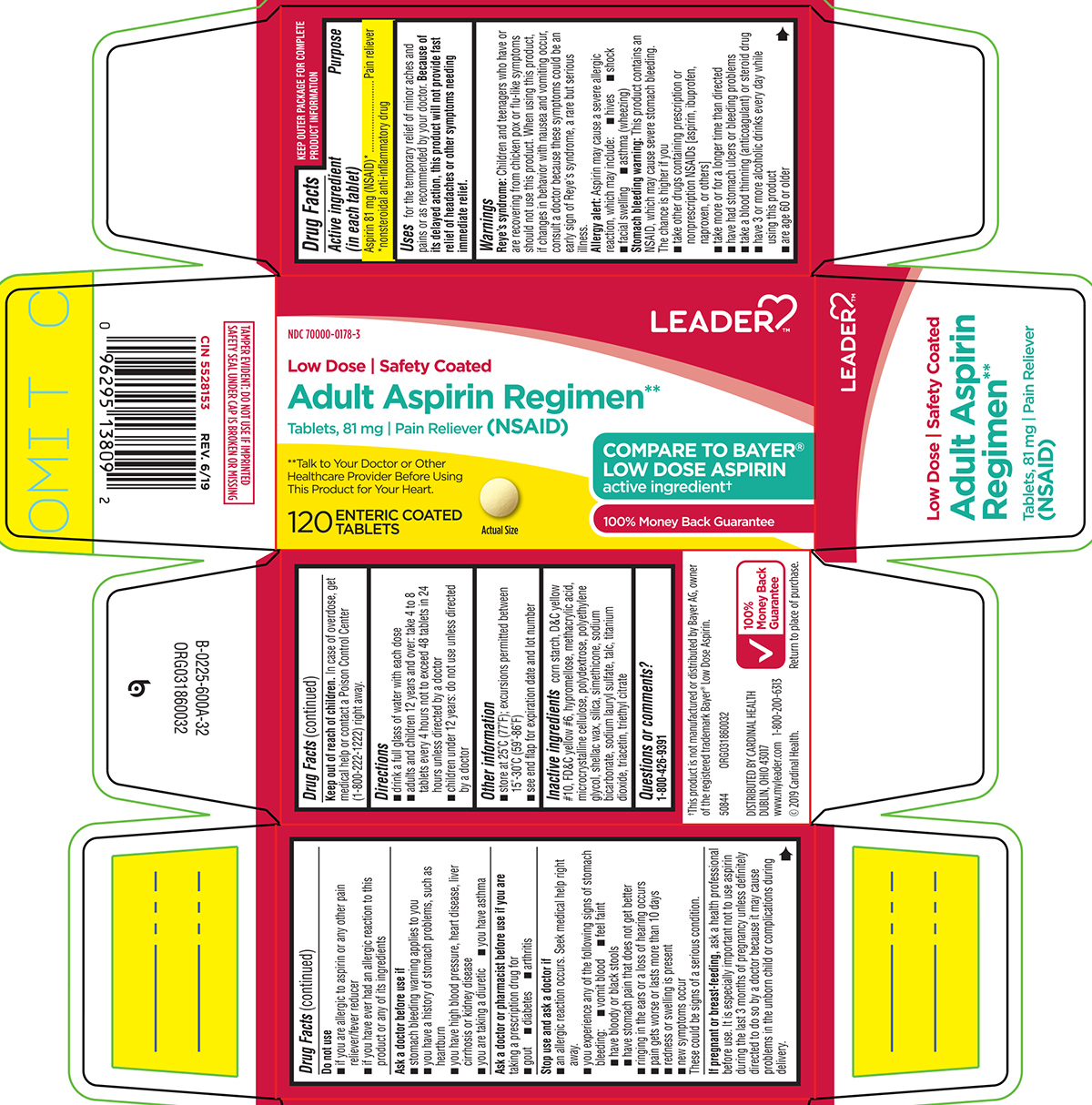
Uses
for the temporary relief of minor aches and pains or as recommended by your doctor. Because of its delayed action, this product will not provide fast relief of headaches or other symptoms needing immediate relief.
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- hives
- shock
- facial swelling
- asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- take more or for a longer time than directed
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- have 3 or more alcoholic drinks every day while using this product
- are age 60 or older
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are
taking a prescription drug for
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- vomit blood
- feel faint
- have bloody or black stools
- have stomach pain that does not get better
- ringing in the ears or a loss of hearing occurs
- pain gets worse or lasts more than 10 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
Directions
- drink a full glass of water with each dose
- adults and children 12 years and over: take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours unless directed by a doctor
- children under 12 years: do not use unless directed by a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10, FD&C yellow #6, hypromellose, methacrylic acid, microcrystalline cellulose, polydextrose, polyethylene glycol, shellac wax, silica, simethicone, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triacetin, triethyl citrate
Principal display panel
LEADER™
NDC 70000-0178-3
Low Dose | Safety Coated
Adult Aspirin Regimen**
Tablets, 81 mg | Pain Reliever (NSAID)
**Talk to Your Doctor or Other Healthcare Provider Before Using This Product for Your Heart.
120
ENTERIC COATED
TABLETS
Actual Size
COMPARE TO BAYER® LOW DOSE ASPIRIN active ingredient†
100% Money Back Guarantee
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
CIN 5528153 REV. 6/19
†This product is not manufactured or distributed by Bayer AG, owner of the registered trademark Bayer® Low Dose Aspirin.
50844 ORG031860032
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OH 43017
www.myleader.com 1-800-200-6313
© 2019 Cardinal Health.
100% Money Back Guarantee
Return to place of purchase.
Leader 44-600A