Ethacrynic acid is a potent diuretic which, if given in excessive amounts, may lead to profound diuresis with water and electrolyte depletion. Therefore, careful medical supervision is required, and dose and dose schedule must be adjusted to the individual patient's needs (see DOSAGE AND ADMINISTRATION).
DESCRIPTION
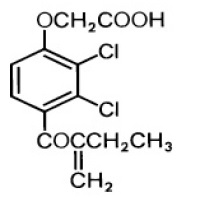
Ethacrynic acid is an unsaturated ketone derivative of an aryloxyacetic acid. It is designated chemically as [2,3-dichloro-4-(2-methylene-1-oxobutyl)phenoxy] acetic acid, and has a molecular weight of 303.14. Ethacrynic acid is a white, or practically white, odor less or practically odorless, crystalline powder, freely soluble in alcohol, chloroform and ether, very slightly soluble in water. Its empirical formula is C13H12Cl2O4 and its structural formula is:
Ethacrynic Acid Tablets USP are supplied as 25 mg tablets for oral use. The tablets contain the following inactive ingredients: lactose monohydrate, pregelatinized starch (corn), colloidal silicon dioxide, talc and calcium stearate.
FDA approved dissolution specification differs from the USP dissolution specification.
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
Ethacrynic acid acts on the ascending limb of the loop of Henle and on the proximal and distal tubules. Urinary output is usually dose dependent and related to the magnitude of fluid accumulation. Water and electrolyte excretion may be increased several times over that observed with thiazide diuretics, since ethacrynic acid inhibits reabsorption of a much greater proportion of filtered sodium than most other diuretic agents. Therefore, ethacrynic acid is effective in many patients who have significant degrees of renal insufficiency (see WARNINGS
concerning deafness). Ethacrynic acid has little or no effect on glomerular filtration or on renal blood flow, except following pronounced reductions in plasma volume when associated with rapid diuresis.
The electrolyte excretion pattern of ethacrynic acid varies from that of the thiazides and mercurial diuretics. Initial sodium and chloride excretion is usually substantial and chloride loss exceeds that of sodium. With prolonged administration, chloride excretion declines, and potassium and hydrogen ion excretion may increase. Ethacrynic acid is effective whether or not there is clinical acidosis or alkalosis.
Although ethacrynic acid, in carefully controlled studies in animals and experimental subjects, produces a more favorable sodium/potassium excretion ratio than the thiazides, in patients with increased diuresis excessive amounts of potassium may be excreted.
Onset of action is rapid, usually within 30 minutes after an oral dose of ethacrynic acid. After oral use, diuresis peaks in about 2 hours and lasts about 6 to 8 hours.
The sulfhydryl binding propensity of ethacrynic acid differs somewhat from that of the organomercurials. Its mode of action is not by carbonic anhydrase inhibition.
Ethacrynic acid does not cross the blood-brain barrier.
INDICATIONS AND USAGE
Ethacrynic Acid Tablets, USP are indicated for treatment of edema when an agent with greater diuretic potential than those commonly employed is required.
- Treatment of the edema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including the nephrotic syndrome.
- Short-term management of ascites due to malignancy, idiopathic edema, and lymphedema.
- Short-term management of hospitalized pediatric patients, other than infants, with congenital heart disease or the nephrotic syndrome.
CONTRAINDICATIONS
All diuretics, including ethacrynic acid, are contraindicated in anuria. If increasing electrolyte imbalance, azotemia, and/or oliguria occur during treatment of severe, progressive renal disease, the diuretic should be discontinued.
In a few patients this diuretic has produced severe, watery diarrhea. If this occurs, it should be discontinued and not used again.
Until further experience in infants is accumulated, therapy with oral and parenteral ethacrynic acid is contraindicated.
Hypersensitivity to any component of this product.
WARNINGS
The effects of ethacrynic acid on electrolytes are related to its renal pharmacologic activity and are dose dependent. The possibility of profound electrolyte and water loss may be avoided by weighing the patient throughout the treatment period, by careful adjustment of dosage, by initiating treatment with small doses, and by using the drug on an intermittent schedule when possible. When excessive diuresis occurs, the drug should be withdrawn until homeostasis is restored. When excessive electrolyte loss occurs, the dosage should be reduced or the drug temporarily withdrawn.
Initiation of diuretic therapy with ethacrynic acid in the cirrhotic patient with ascites is best carried out in the hospital. When maintenance therapy has been established, the individual can be satisfactorily followed as an outpatient.
Ethacrynic acid should be given with caution to patients with advanced cirrhosis of the liver, particularly those with a history of previous episodes of electrolyte imbalance or hepatic encephalopathy. Like other diuretics it may precipitate hepatic coma and death.
Too vigorous a diuresis, as evidenced by rapid and excessive weight loss, may induce an acute hypotensive episode. In elderly cardiac patients, rapid contraction of plasma volume and the resultant hemoconcentration should be avoided to prevent the development of thromboembolic episodes, such as cerebral vascular thromboses and pulmonary emboli which may be fatal. Excessive loss of potassium in patients receiving digitalis glycosides may precipitate digitalis toxicity. Care should also be exercised in patients receiving potassium-depleting steroids.
A number of possibly drug-related deaths have occurred in critically ill patients refractory to other diuretics. These generally have fallen into two categories: (1) patients with severe myocardial disease who have been receiving digitalis and presumably developed acute hypokalemia with fatal arrhythmia; (2) patients with severely decompensated hepatic cirrhosis with ascites, with or without accompanying encephalopathy, who were in electrolyte imbalance and died because of intensification of the electrolyte defect.
Deafness, tinnitus, and vertigo with a sense of fullness in the ears have occurred, most frequently in patients with severe impairment of renal function. These symptoms have been associated most often with intravenous administration and with doses in excess of those recommended. The deafness has usually been reversible and of short duration (one to 24 hours). However, in some patients the hearing loss has been permanent. A number of these patients were also receiving drugs known to be ototoxic. Ethacrynic acid may increase the ototoxic potential of other drugs (see PRECAUTIONS, Drug Interactions).
Lithium generally should not be given with diuretics (see PRECAUTIONS, Drug Interactions).
PRECAUTIONS
General
Weakness, muscle cramps, paresthesias, thirst, anorexia, and signs of hyponatremia, hypokalemia, and/or hypochloremic alkalosis may occur following vigorous or excessive diuresis and these may be accentuated by rigid salt restriction. Rarely, tetany has been reported following vigorous diuresis. During therapy with ethacrynic acid, liberalization of salt intake and supplementary potassium chloride are often necessary.
When a metabolic alkalosis may be anticipated, e.g., in cirrhosis with ascites, the use of potassium chloride or a potassium-sparing agent before and during therapy with ethacrynic acid may mitigate or prevent the hypokalemia.
Loop diuretics have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
The safety and efficacy of ethacrynic acid in hypertension have not been established. However, the dosage of coadministered antihypertensive agents may require adjustment.
Orthostatic hypotension may occur in patients receiving other antihypertensive agents when given ethacrynic acid.
Ethacrynic acid has little or no effect on glomerular filtration or on renal blood flow, except following pronounced reductions in plasma volume when associated with rapid diuresis. A transient increase in serum urea nitrogen may occur. Usually, this is readily reversible when the drug is discontinued.
As with other diuretics used in the treatment of renal edema, hypoproteinemia may reduce responsiveness to ethacrynic acid and the use of salt-poor albumin should be considered.
A number of drugs, including ethacrynic acid, have been shown to displace warfarin from plasma protein; a reduction in the usual anticoagulant dosage may be required in patients receiving both drugs.
Ethacrynic acid may increase the risk of gastric hemorrhage associated with corticosteroid treatment.
Laboratory Tests
Frequent serum electrolyte, CO2 and BUN determinations should be performed early in therapy and periodically thereafter during active diuresis. Any electrolyte abnormalities should be corrected or the drug temporarily withdrawn.
Increases in blood glucose and alterations in glucose tolerance tests have been observed in patients receiving ethacrynic acid.
Drug Interactions
Lithium generally should not be given with diuretics because they reduce its renal clearance and add a high risk of lithium toxicity. Read circulars for lithium preparations before use of such concomitant therapy.
Ethacrynic acid may increase the ototoxic potential of other drugs such as aminoglycoside and some cephalosporin antibiotics. Their concurrent use should be avoided.
A number of drugs, including ethacrynic acid, have been shown to displace warfarin from plasma protein; a reduction in the usual anticoagulant dosage may be required in patients receiving both drugs.
In some patients, the administration of a non-steroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics. Therefore, when ethacrynic acid and non-steroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of a tumorigenic effect in a 79-week oral chronic toxicity study in rats at doses up to 45 times the human dose.
Ethacrynic acid had no effect on fertility in a two-litter study in rats or a two-generation study in mice at 10 times the human dose.
Pregnancy
Pregnancy Category B: Reproduction studies in the mouse and rabbit at doses up to 50 times the human dose showed no evidence of external abnormalities of the fetus due to ethacrynic acid.
In a two-litter study in the dog and rat, oral doses of 5 mg/kg/day or 20 mg/kg/day (2½ or 10 times the human dose), respectively, did not interfere with pregnancy or with growth and development of the pups. Although there was reduction in the mean body weights of the fetuses in a teratogenic study in the rat at a dose level of 100 mg/kg (50 times the human dose), there was no effect on mortality or postnatal development. Functional and morphologic abnormalities were not observed.
There are, however, no adequate and well-controlled studies in pregnant women. Since animal reproduction studies are not always predictive of human response, ethacrynic acid should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ethacrynic acid, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There are no well-controlled clinical trials in pediatric patients. The information on oral dosing in pediatric patients, other than infants, is supported by evidence from empiric use in this age group.
For information on oral use in pediatric patients, other than infants, see INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION.
Safety and effectiveness of oral and parenteral use in infants have not been established (see CONTRAINDICATIONS).
Geriatric Use
Of the total number of subjects in clinical studies of ethacrynic acid, approximately 224 patients (21%) were 65 to 74 years of age, while approximately 100 patients (9%) were 75 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. (See WARNINGS.)
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. (See CONTRAINDICATIONS.)
ADVERSE REACTIONS
Gastrointestinal
Anorexia, malaise, abdominal discomfort or pain, dysphagia, nausea, vomiting, and diarrhea have occurred. These are more frequent with large doses or after one to three months of continuous therapy. A few patients have had sudden onset of profuse, watery diarrhea. Discontinue Ethacrynic Acid Tablets, USP if diarrhea is severe and do not give it again. Gastrointestinal bleeding has occurred in some patients. Rarely, acute pancreatitis has been reported.
Metabolic
Reversible hyperuricemia and acute gout have been reported. Acute symptomatic hypoglycemia with convulsions occurred in two uremic patients who received doses above those recommended. Hyperglycemia has been reported. Rarely, jaundice and abnormal liver function tests have been reported in seriously ill patients receiving multiple drug therapy, including Ethacrynic Acid Tablets, USP.
Hematologic
Agranulocytosis or severe neutropenia has been reported in a few critically ill patients also receiving agents known to produce this effect. Thrombocytopenia has been reported rarely. Henoch-Schönlein purpura has been reported rarely in patients with rheumatic heart disease receiving multiple drug therapy, including Ethacrynic Acid Tablets, USP.
Special Senses (see WARNINGS)
Deafness, tinnitus and vertigo with a sense of fullness in the ears, and blurred vision have occurred.
Central Nervous System
Headache, fatigue, apprehension, confusion.
Miscellaneous
Skin rash, fever, chills, hematuria.
OVERDOSAGE
Overdosage may lead to excessive diuresis with electrolyte depletion and dehydration.
In the event of overdosage, symptomatic and supportive measures should be employed. Emesis should be induced or gastric lavage performed. Correct dehydration, electrolyte imbalance, hepatic coma, and hypotension by established procedures. If required, give oxygen or artificial respiration for respiratory impairment.
In the mouse, the oral LD50 of ethacrynic acid is 627 mg/kg.
DOSAGE AND ADMINISTRATION
Dosage must be regulated carefully to prevent a more rapid or substantial loss of fluid or electrolyte than is indicated or necessary. The magnitude of diuresis and natriuresis is largely dependent on the degree of fluid accumulation present in the patient. Similarly, the extent of potassium excretion is determined in large measure by the presence and magnitude of aldosteronism.
Oral Use
Ethacrynic Acid Tablets, USP are available for oral use as 25 mg tablets.
Dosage: To Initiate Diuresis
In Adults: The smallest dose required to produce gradual weight loss (about 1 pound to 2 pounds per day) is recommended. Onset of diuresis usually occurs at 50 mg to 100 mg for adults. After diuresis has been achieved, the minimally effective dose (usually from 50 mg to 200 mg daily) may be given on a continuous or intermittent dosage schedule. Dosage adjustments are usually in 25 mg to 50 mg increments to avoid derangement of water and electrolyte excretion.
The patient should be weighed under standard conditions before and during the institution of diuretic therapy with this compound. Small alterations in dose should effectively prevent a massive diuretic response. The following schedule may be helpful in determining the smallest effective dose.
- Day 1 — 50 mg once daily after a meal
- Day 2 — 50 mg twice daily after meals, if necessary
- Day 3 — 100 mg in the morning and 50 mg to 100 mg following the afternoon or evening meal, depending upon response to the morning dose.
A few patients may require initial and maintenance doses as high as 200 mg twice daily. These higher doses, which should be achieved gradually, are most often required in patients with severe, refractory edema.
In Pediatric Patients (excluding infants, see CONTRAINDICATIONS): The initial dose should be 25 mg. Careful stepwise increments in dosage of 25 mg should be made to achieve effective maintenance.
Maintenance Therapy
It is usually possible to reduce the dosage and frequency of administration once dry weight has been achieved.
Ethacrynic Acid Tablets, USP may be given intermittently after an effective diuresis is obtained with the regimen outlined above. Dosage may be on an alternate daily schedule or more prolonged periods of diuretic therapy may be interspersed with rest periods. Such an intermittent dosage schedule allows time for correction of any electrolyte imbalance and may provide a more efficient diuretic response.
The chloruretic effect of this agent may give rise to retention of bicarbonate and a metabolic alkalosis. This may be corrected by giving chloride (ammonium chloride or arginine chloride). Ammonium chloride should not be given to cirrhotic patients.
Ethacrynic Acid Tablets, USP has additive effects when used with other diuretics. For example, a patient who is on maintenance dosage of an oral diuretic may require additional intermittent diuretic therapy, such as an organomercurial, for the maintenance of basal weight. The intermittent use of Ethacrynic Acid Tablets, USP orally may eliminate the need for injections of organomercurials. Small doses of Ethacrynic Acid Tablets, USP may be added to existing diuretic regimens to maintain basal weight. This drug may potentiate the action of carbonic anhydrase inhibitors, with augmentation of natriuresis and kaliuresis. Therefore, when adding ethacrynic acid the initial dose and changes of dose should be in 25 mg increments, to avoid electrolyte depletion. Rarely, patients who failed to respond to ethacrynic acid have responded to older established agents.
While many patients do not require supplemental potassium, the use of potassium chloride or potassium sparing agents, or both, during treatment with Ethacrynic Acid Tablets, USP is advisable, especially in cirrhotic or nephrotic patients and in patients receiving digitalis.
Salt liberalization usually prevents the development of hyponatremia and hypochloremia. During treatment with Ethacrynic Acid Tablets, USP salt may be liberalized to a greater extent than with other diuretics. Cirrhotic patients, however, usually require at least moderate salt restriction concomitant with diuretic therapy.
HOW SUPPLIED
Ethacrynic Acid Tablets, USP 25 mg, are white, capsule shaped scored (functional scoring) tablets debossed with 'SG' on scored side and '334' on the other side and supplied as
Bottles of 30 tablets: NDC 50228-334-30
Bottles of 100 tablets: NDC 50228-334-10
Bottles of 1000 tablets: NDC 50228-334-01
PRINCIPAL DISPLAY PANEL
NDC 50228-334-30
Ethacrynic Acid
Tablets, USP
25 mg
30 Tablets Rx only
ScieGen Pharmaceuticals Inc.

NDC 50228-334-10
Ethacrynic Acid
Tablets, USP
25 mg
100 Tablets Rx only
ScieGen Pharmaceuticals Inc.

NDC 50228-334-01
Ethacrynic Acid
Tablets, USP
25 mg
1,000 Tablets Rx 0nly
ScieGen Pharmaceuticals Inc.
