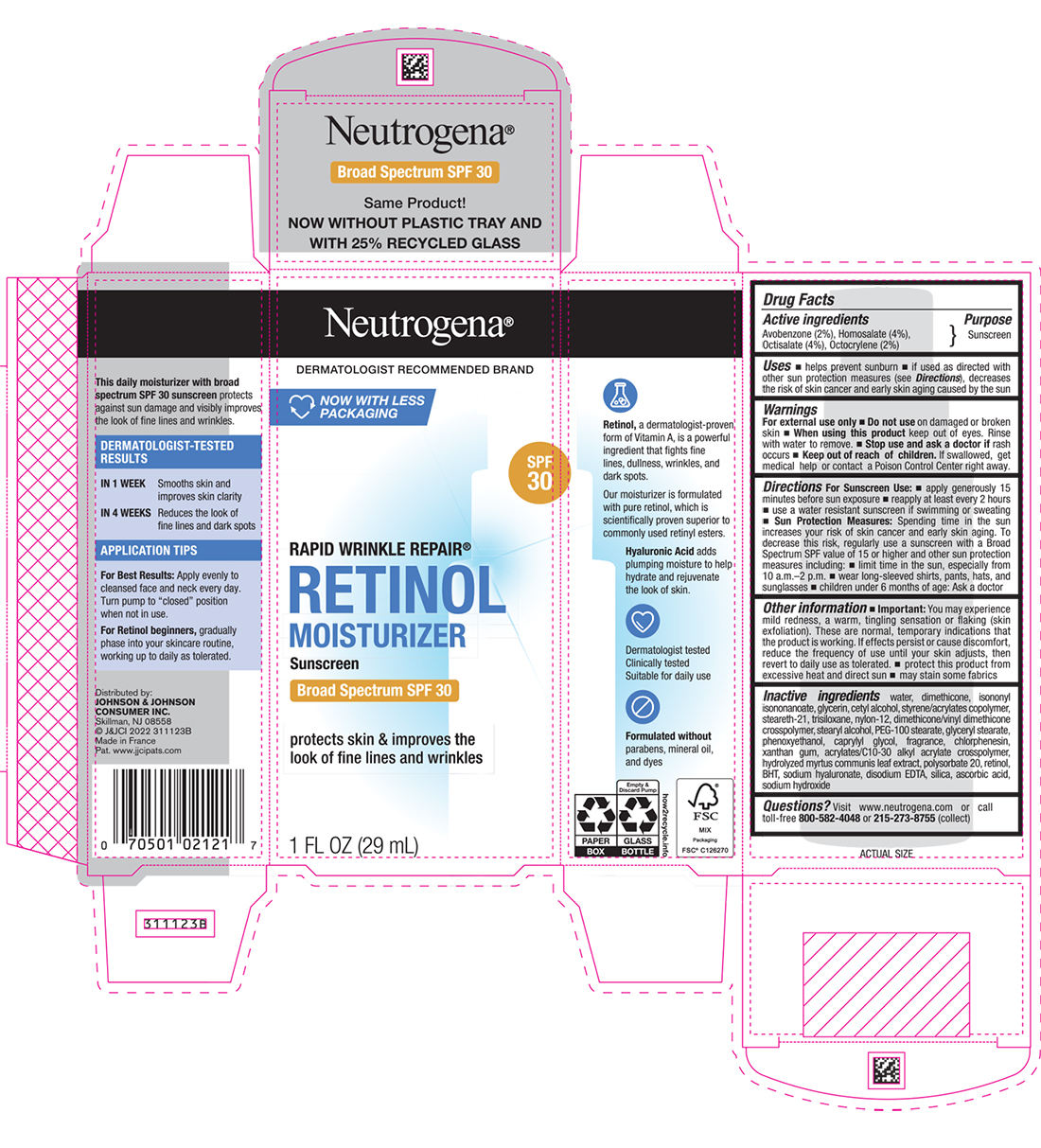
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For Sunscreen Use:
- apply generously 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
Other information
- You may experience mild redness, a warm, tingling sensation or flaking (skin exfoliation). These are normal, temporary indications that the formula is working. If effects persist/cause discomfort, reduce the frequency of use until your skin adjusts, then revert to daily use as tolerated.
- protect this product from excessive heat and direct sun
- may stain some fabrics
Inactive ingredients
water, dimethicone, isononyl isononanoate, glycerin, cetyl alcohol, styrene/acrylates copolymer, steareth-21, trisiloxane, nylon-12, dimethicone/vinyl dimethicone crosspolymer, stearyl alcohol, PEG-100 stearate, glyceryl stearate, phenoxyethanol, caprylyl glycol, fragrance, chlorphenesin, xanthan gum, acrylates/C10-30 alkyl acrylate crosspolymer, hydrolyzed myrtus communis leaf extract, polysorbate 20, retinol, BHT, sodium hyaluronate, disodium EDTA, silica, ascorbic acid, sodium hydroxide