Indication:
Estrogen Receptor Positive Metastatic Breast Cancer
Usual Dosage:
180mg Testosterone & 12mg of Anastrozole
CAUTION:
Federal Law prohibits dispensing without prescription.
Storage:
Store t controlled room temperature
Manufactured by:
ADVANCED PHARMACEUTICAL TECHNOLOGY
Phone: (914)358-5260
Elmsford, NY 10523
Testozole (57377-060-01)
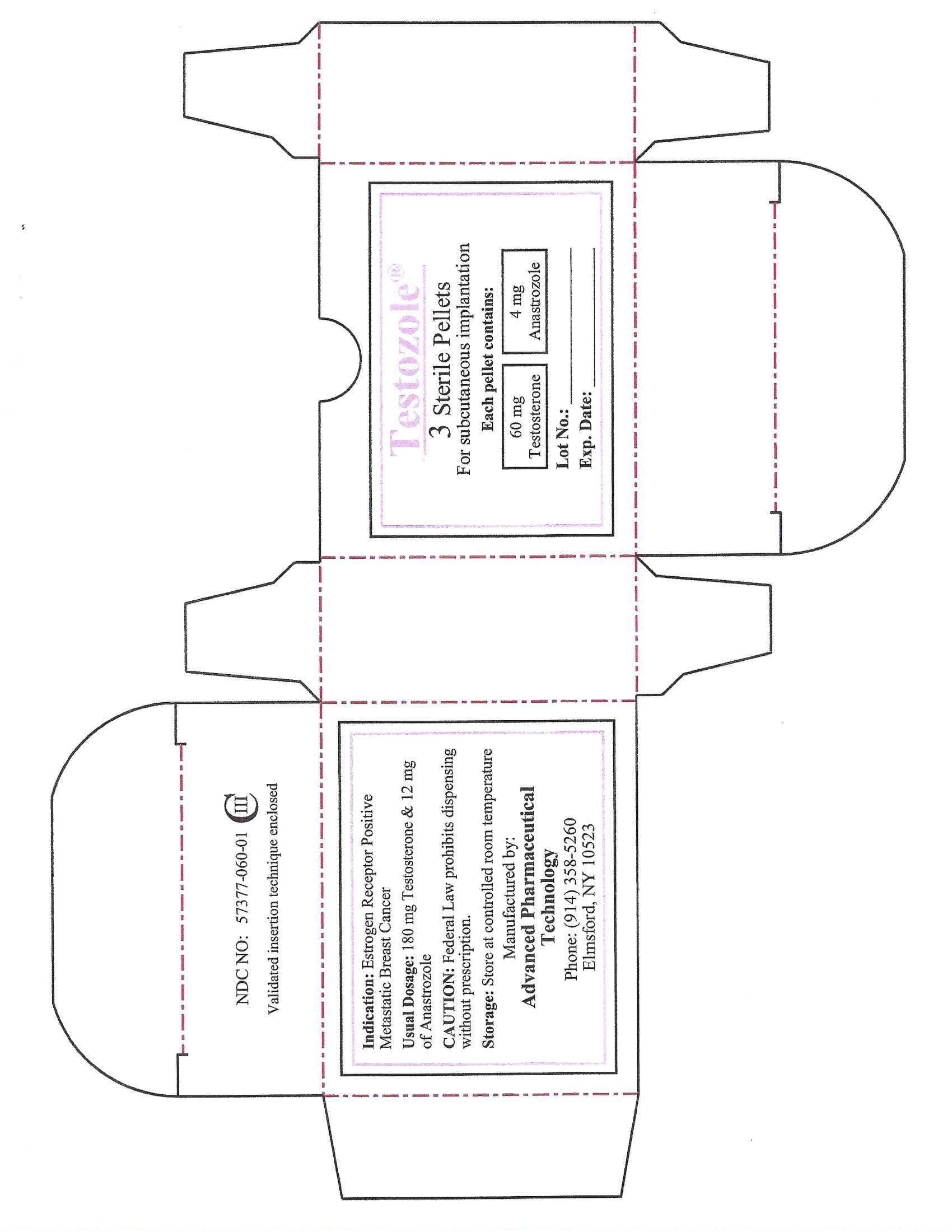
For subcutaneous implantation
Each pellet contains:
60mg Testosterone
4mg Anastrozole
Caution:
Federal Law prohibits dispensing without presciption.
Store at controlled room temperature.
Advanced Pharmaceutical Technology, Inc.