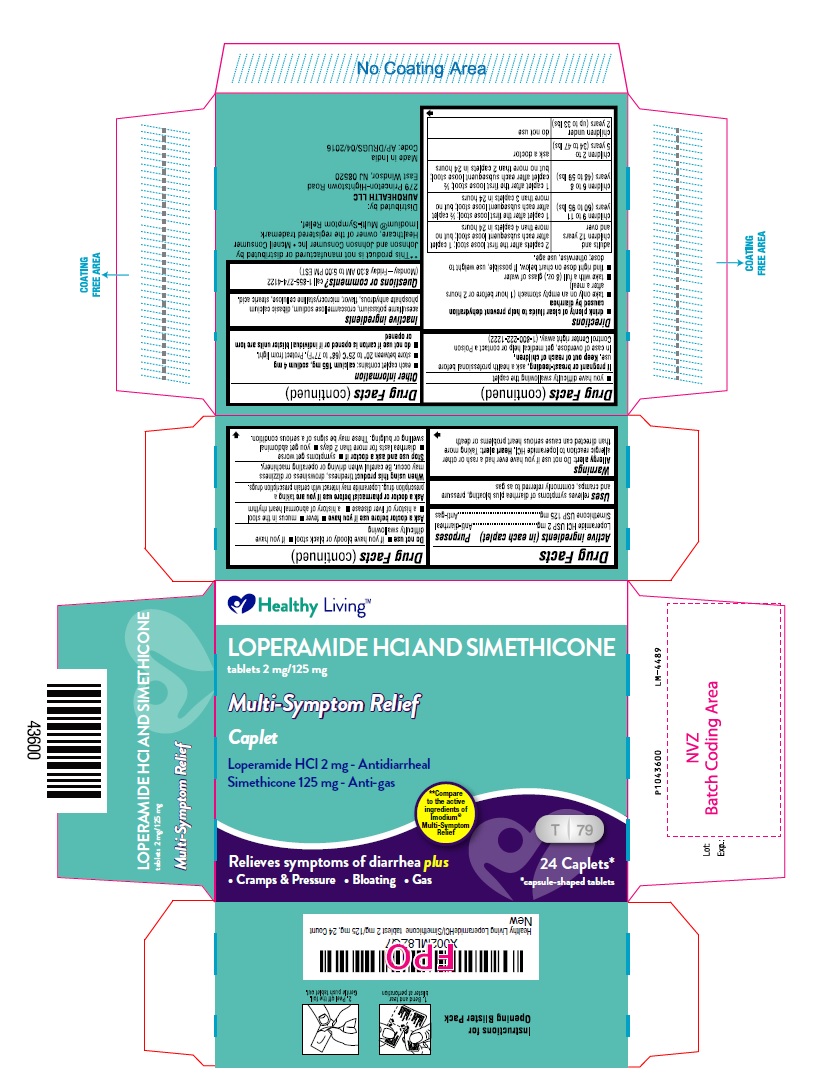
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl
Heart alert: Taking more than directed can cause serious heart problems or death
Ask a doctor before use if you have
- fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product
tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Stop use and ask a doctor if
- symptoms get worse
- diarrhea lasts for more than 2 days
- you get abdominal swelling or bulging. These may be signs of a serious condition.
- you have difficulty swallowing the caplet
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
(1-800-222-1222)
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- take only on an empty stomach (1 hour before or 2 hours after a meal)
- take with a full (8 oz.) glass of water
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
| children 9 to 11 years (60 to 95 lbs) | 1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
| children 6 to 8 years (48 to 59 lbs) | 1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
| children 2 to 5 years (34 to 47 lbs) | ask a doctor |
| children under 2 years (up to 33 lbs) | do not use |
Other information
- each caplet contains: calcium 165 mg, sodium 4 mg
- store between 20° to 25°C (68° to 77°F). Protect from light.
- do not use if carton is opened or if individual blister units are torn or opened
Inactive ingredients
acesulfame potassium, croscarmellose sodium, dibasic calcium phosphate anhydrous, flavor, microcrystalline cellulose, stearic acid.
Questions or comments?
call 1-855-274-4122
(Monday – Friday 8:30 AM to 5:00 PM EST)
Distributed by:
AUROHEALTH LLC
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Made in India
Code: AP/DRUGS/04/2016
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2 mg/125mg (24 Caplets) Blister Carton Label
Healthy Living™
LOPERAMIDE HCl AND SIMETHICONE
tablets 2 mg/125 mg
Multi-Sympton Relief
Caplet
Loperamide HCl 2 mg - Antidiarrheal
Simethicone 125 mg - Anti-gas
** Compare
to the active
ingridients of
Imodium®
Multi-Sympton
Relief
Relieves symptoms of diarrhea plus
• Cramps & Pressure • Bloating • Gas
T 79
24 Caplets*
*capsule-shaped tablets