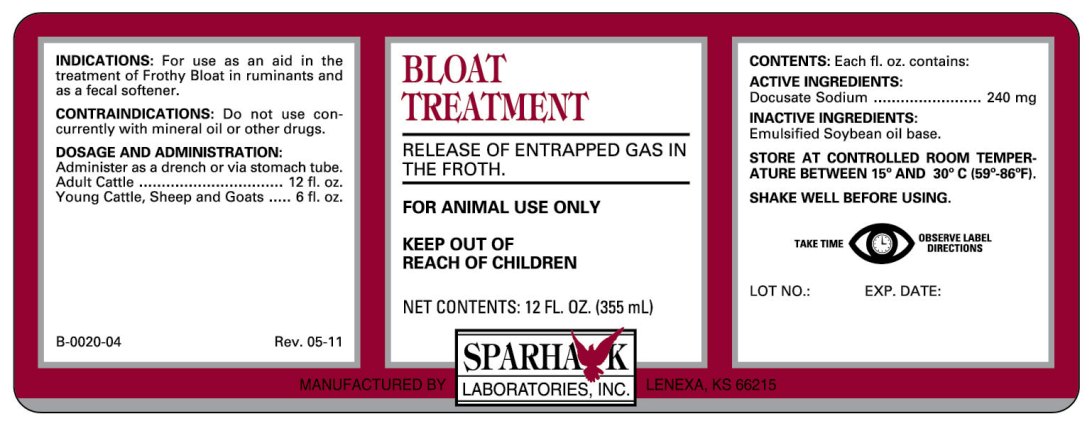
RELEASE OF ENTRAPPED GAS IN THE FROTH
FOR ANIMAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
INDICATIONS
For use as an aid in the treatment of Frothy Bloat in ruminants and as a fecal softener.
DOSAGE AND ADMINISTRATION
Administer as a drench or via stomach tube.
Adult Cattle................................. 12 fl. oz.
Young Cattle, Sheep and Goats ..... 6 fl. oz.
CONTENTS
Each fl. oz. contains:
ACTIVE INGREDIENTS:
Docusate Sodium ................. 240 mg
INACTIVE INGREDIENTS:
Emulsified Soybean oil base.