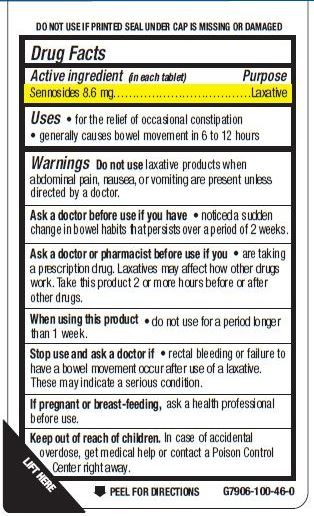
Warnings
Do not use laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
Ask a doctor before use if you have
- noticed a sudden change in bowel habits that persists over a period of 2 weeks.
Ask a doctor or pharmacist before use if you
- are taking a prescription drug. Laxatives may affect how other drugs work. Take this product 2 or more hours before or after
other drugs.
When using this product
- do not use for a period longer than 1 week.
Stop use and ask a doctor if
- rectal bleeding or failure to have a bowel movement occur after use of a laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of accidental overdose, get medical help or contact a Poison Control Center right away.
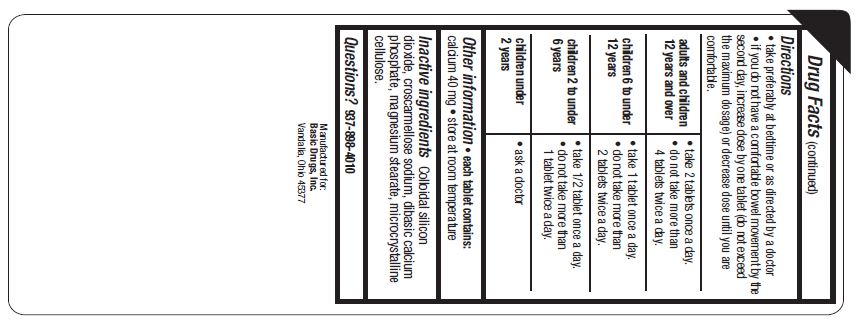
Directions
- take preferably at bedtime or as directed by a doctor
- If you do not have a confortable bowel movement by the second day, increase dosage by one tablet (do not exceed the maximum dosage) or decrease dose until you are confortable.
| adults and children 12 years and over |
|
| children 6 to under 12 years |
|
| children 2 to under 6 years |
|
| children under 2 years |
|