USE
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
On damages or broken skin Keep out of eyes. Rinse with water to remove. If rash occurs
FOR EXTERNALS USE ONLYDO NOT USEWHEN USING THIS PRODUCTSTOP USE AND ASK A DOCTOR
Keep Out Of Reach Of Children.
If swallowed, get medical help or contact Poison Control Center right away.
DIRECTIONS
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months of age: Ask a doctor
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
-
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
OTHER INFORMATION
- Protect the product in this container from excessive heat and direct sun
- Do not use if carton is open or if seal underneath tube cap is missing or broke
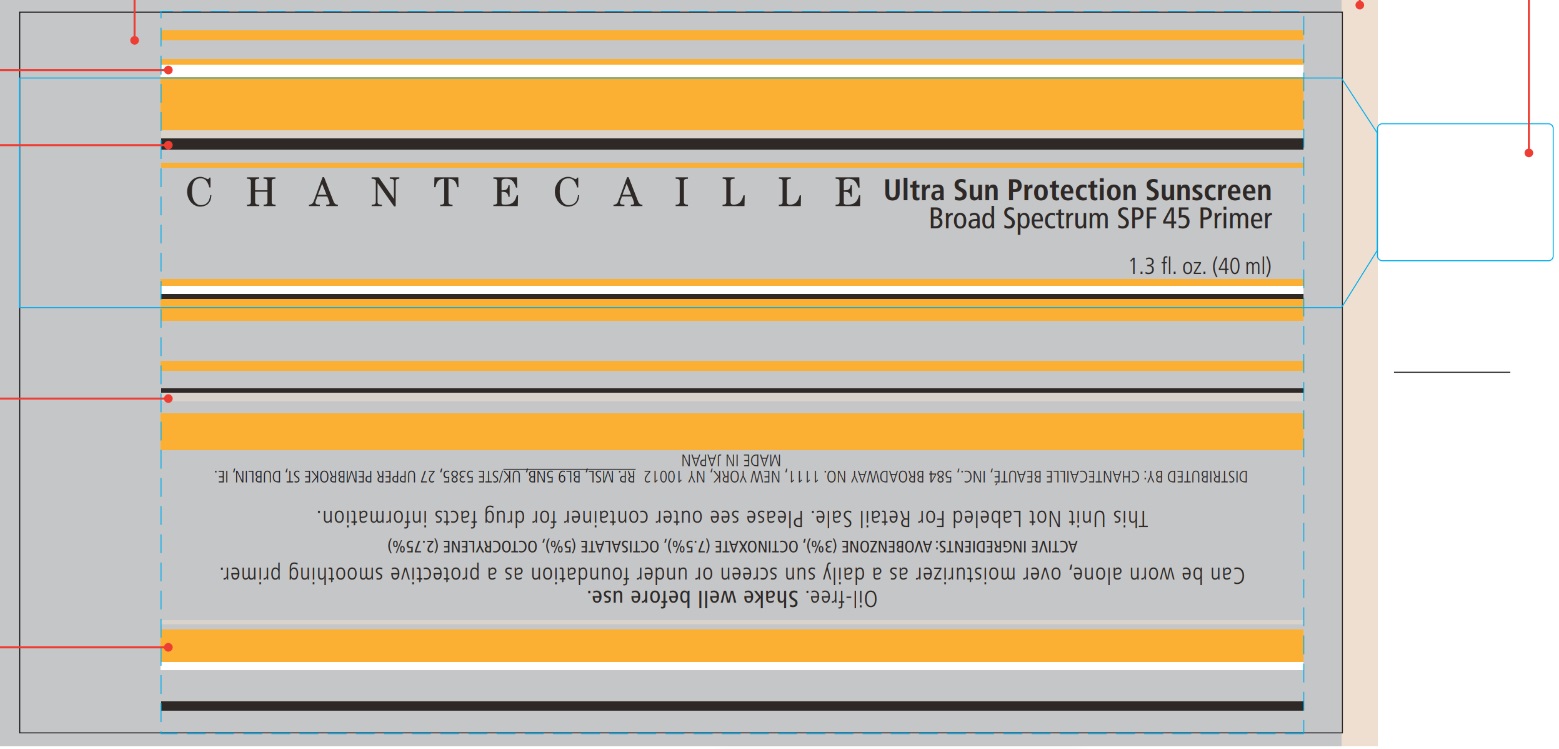
INACTIVE INGREDIENTS:
DAMASK ROSE (ROSA DAMASCENA) FLOWER WATER, C12-15 ALKYL BENZOATE, BUTYLENE GLYCOL, POLYMETHYLSILSESQUIOXANE, POLYMETHYL METHACRYLATE, ISONONYL ISONONANOATE, METHYL METHACRYLATE CROSSPOLYMER, GLYCERIN, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, DICAPRYLYL CARBONATE, PENTYLENE GLYCOL, POLYGLYCERYL-6 POLYRICINOLEATE, PHENOXYETHANOL, SODIUM CHLORIDE, POLYGLYCERYL-2 ISOSTEARATE, SORBITAN SESQUIISOSTEARATE, TETRASODIUM EDTA, TOCOPHEROL, LEMON BALM (MELISSA OFFICINALIS) LEAF EXTRACT, STEARYL GLYCYRRHETINATE, CARNOSINE, LECITHIN, TEA (CAMELLIA SINENSIS) LEAF EXTRACT, YOSHINO CHERRY (PRUNUS YEDOENSIS) LEAF EXTRACT, CYCLODEXTRIN