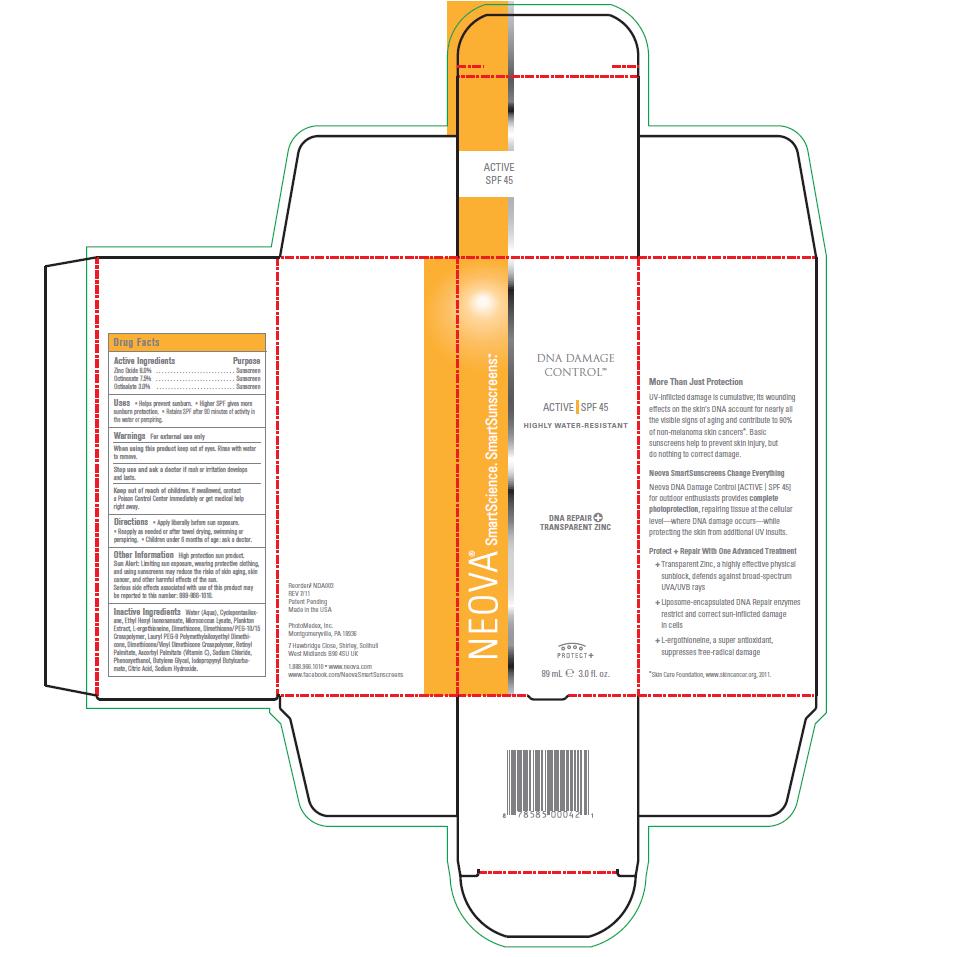
Uses
• Helps prevent sunburn. • Higher SPF gives more
sunburn protection. • Retains SPF after 80 minutes of activity in
the water or perspiring.
Keep out of reach of children
If swallowed, contact a Poison Control Center immediately or get medical help right away.
Directions
Apply liberally before sun exposure.
• Reapply as needed or after towel drying, swimming or perspiring. • Children under 6 months of age: ask a doctor.
Other Information
High protection sun product. Sun Alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun. Serious side effects associated with use of this product may be reported to this number: 888-966-1010.
Inactive Ingredients
Water (Aqua), Cyclopentasiloxane,
Ethyl Hexyl Isononanoate, Micrococcus Lysate, Plankton
Extract, L-ergothioneine, Dimethicone, Dimethicone/PEG-10/15
Crosspolymer, Lauryl PEG-9 Polymethylsiloxyethyl Dimethicone,
Dimethicone/Vinyl Dimethicone Crosspolymer, Retinyl
Palmitate, Ascorbyl Palmitate (Vitamin C), Sodium Chloride,
Phenoxyethanol, Butylene Glycol, Iodopropynyl Butylcarbamate,
Citric Acid, Sodium Hydroxide.