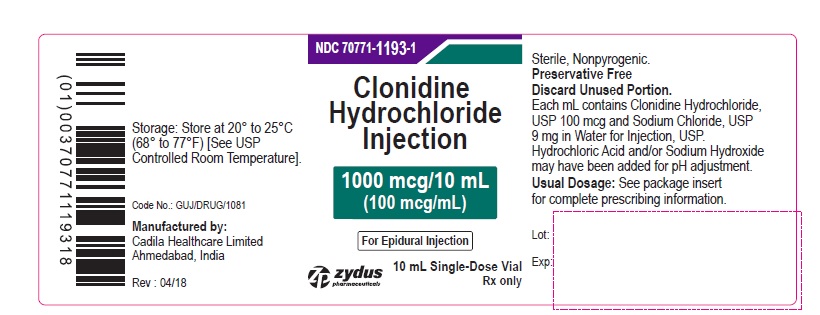
PRINCIPAL DISPLAY PANEL – 100 mcg/mL Container Label
NDC 70771-1193-1
Clonidine Hydrochloride Injection
1000 mcg/10 mL
(100 mcg/mL)
For Epidural Injection
Rx only
10 mL Single-Dose Vial
Zydus pharmaceuticals
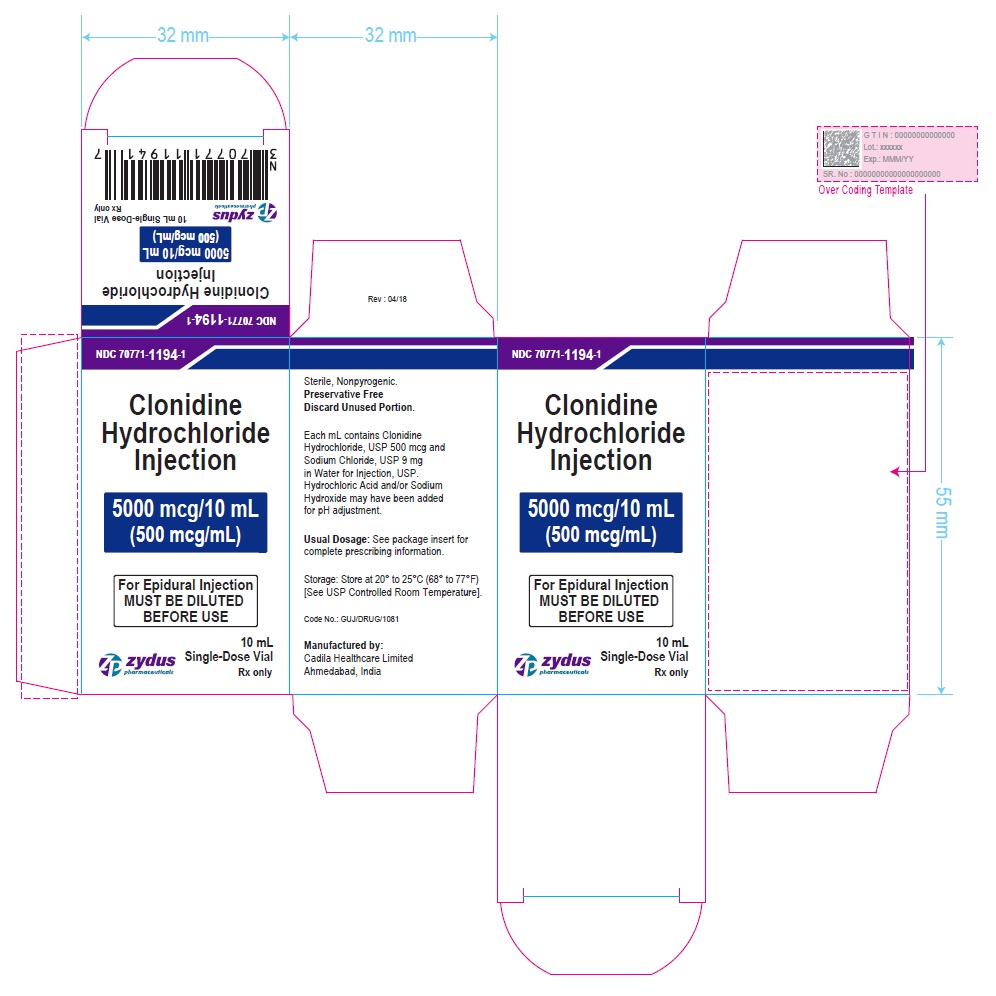
PRINCIPAL DISPLAY PANEL – 100 mcg/mL Carton Label
Rx only
Zydus pharmaceuticals
NDC 70771-1193-1
Clonidine Hydrochloride Injection
1000 mcg/10 mL
(100 mcg/mL)
For Epidural Injection
10 mL Single-Dose Vial
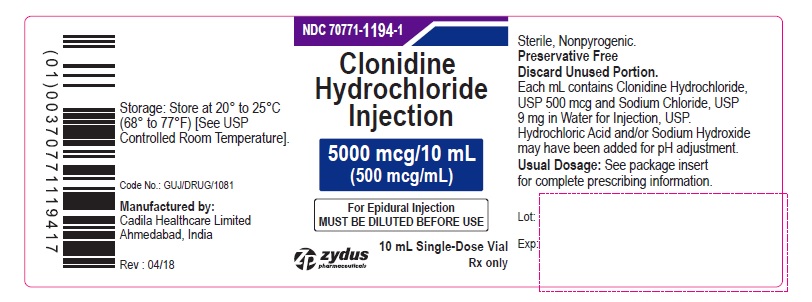
PRINCIPAL DISPLAY PANEL – 500 mcg/mL Container Label
NDC 70771-1194-1
Clonidine Hydrochloride Injection
5000 mcg/10 mL
(500 mcg/mL)
For Epidural Injection
MUST BE DILUTED BEFORE USE
Rx only
10 mL Single-Dose Vial
Zydus pharmaceuticals
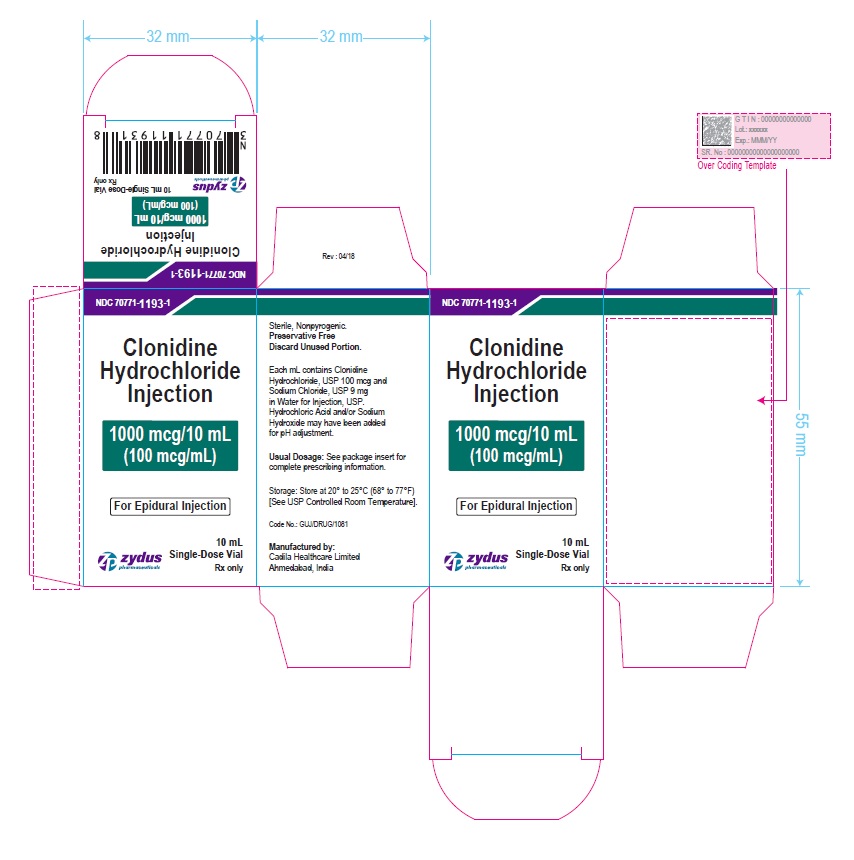
PRINCIPAL DISPLAY PANEL – 500 mcg/mL Carton Label
Rx only
Zydus pharmaceuticals
NDC 70771-1194-1
Clonidine Hydrochloride Injection
5000 mcg/10 mL
(500 mcg/mL)
For Epidural Injection
MUST BE DILUTED BEFORE USE
10 mL Single-Dose Vial