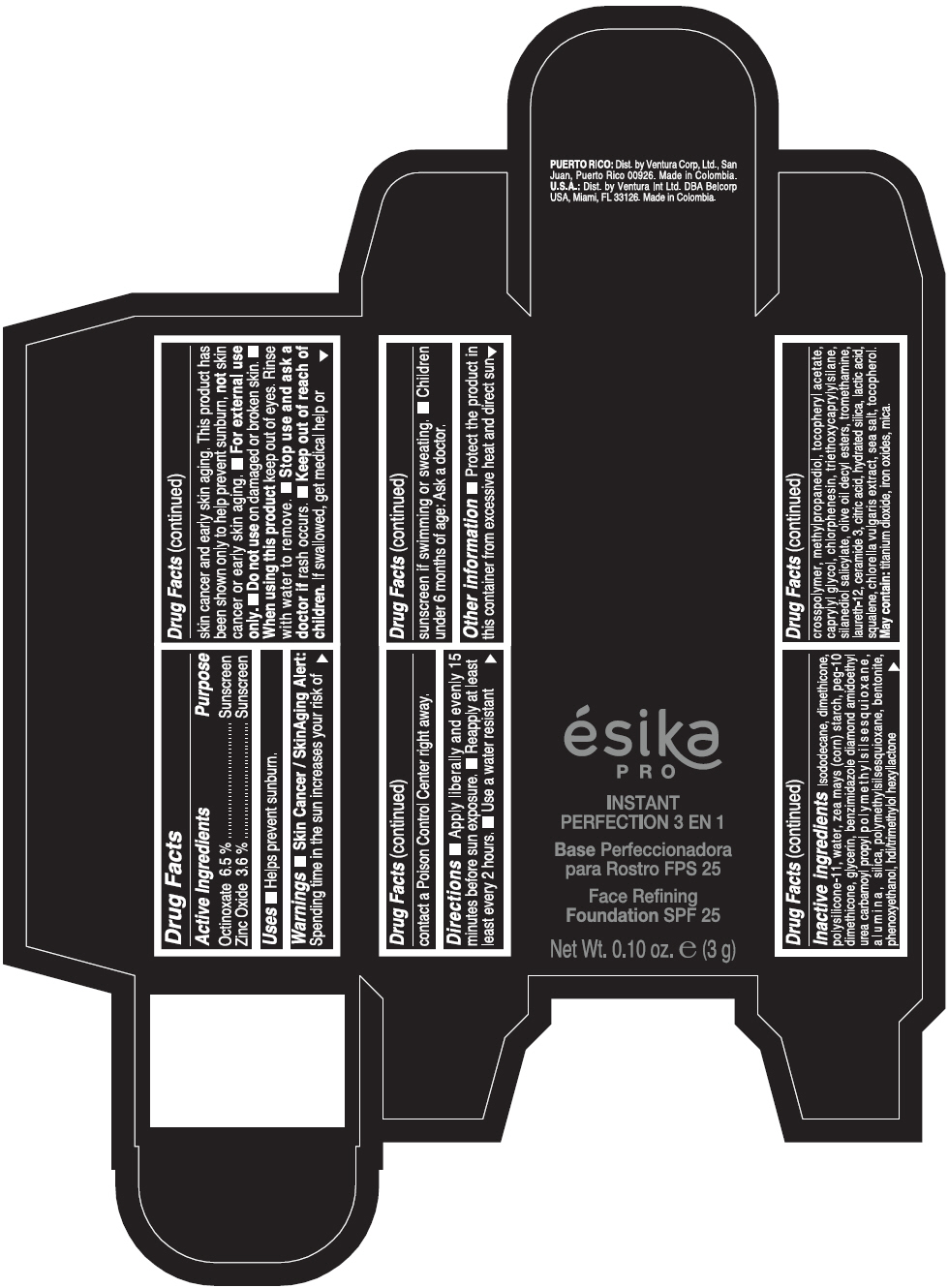
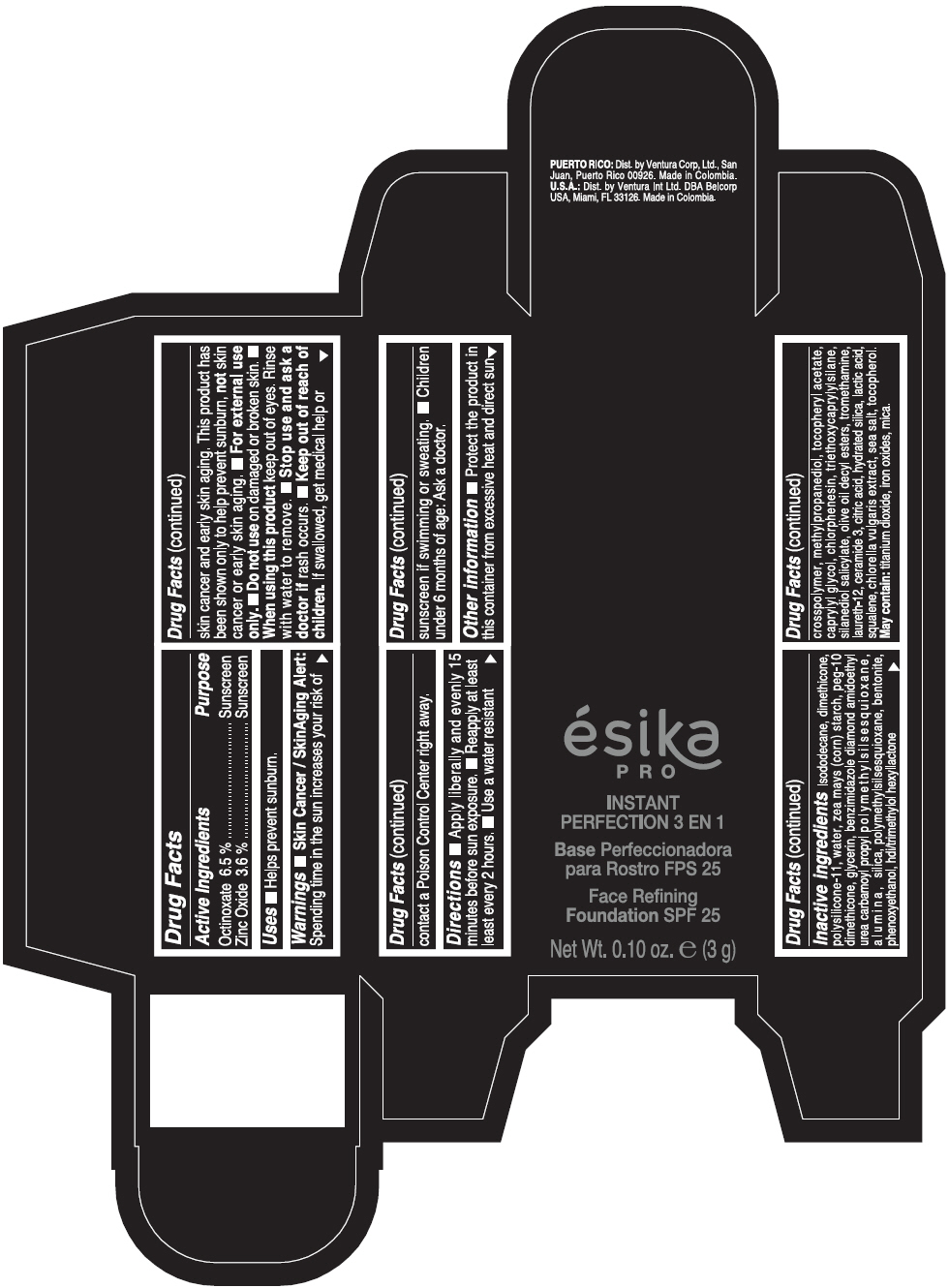
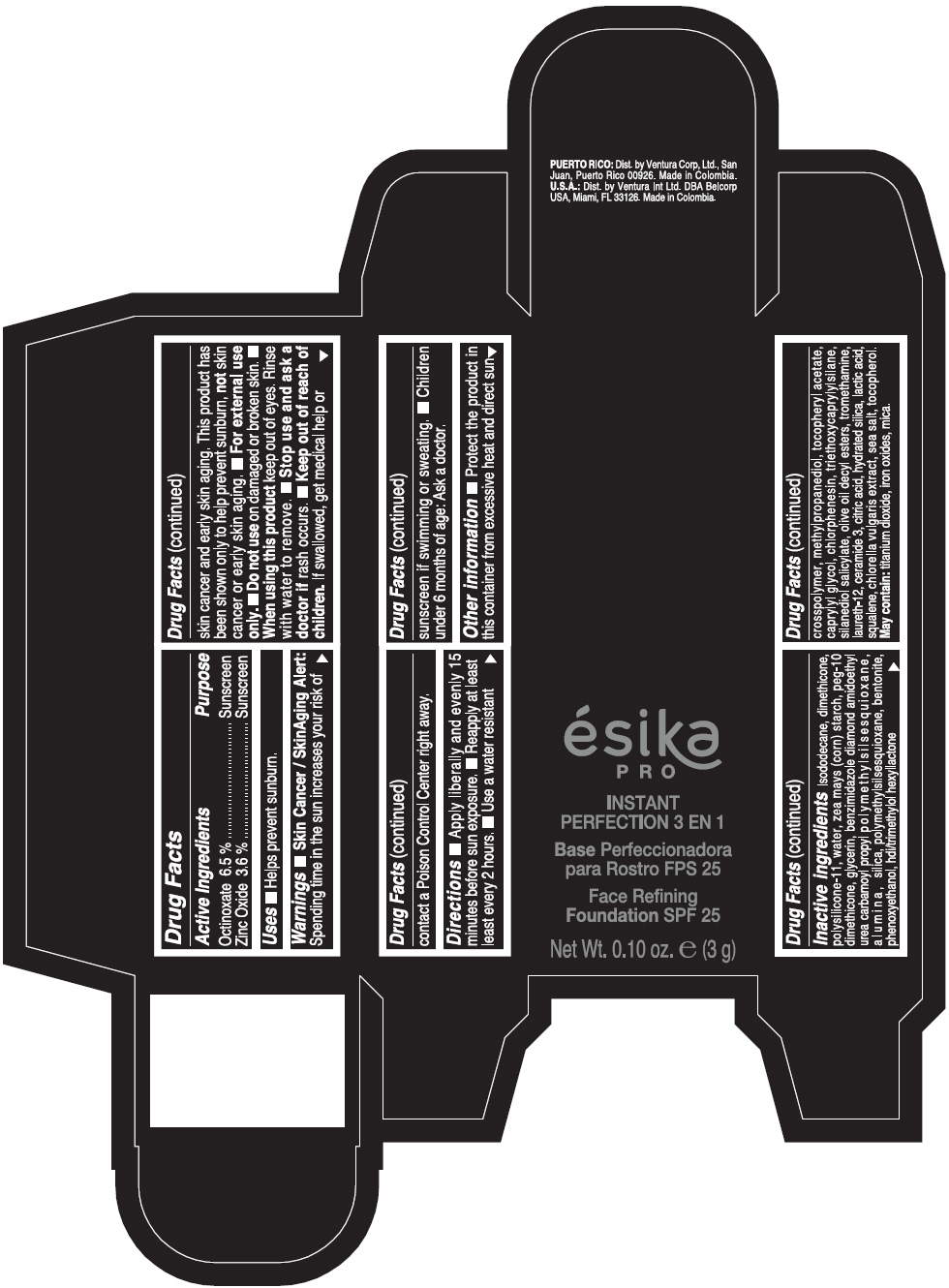
Warnings
- Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
- For external use only.
Directions
- Apply liberally and evenly 15 minutes before sun exposure. Reapply at least every 2 hours. Use a water resistant sunscreen if swimming or sweating. Children under 6 months of age: Ask a doctor.
Inactive ingredients
ISODODECANE, DIMETHICONE, POLYSILICONE-11, WATER, ZEA MAYS (CORN) STARCH, PEG-10 DIMETHICONE, GLYCERIN, BENZIMIDAZOLE DIAMOND AMIDOETHYL UREA CARBAMOYL PROPYL POLYMETHYLSILSESQUIOXANE, ALUMINA, SILICA, POLYMETHYLSILSESQUIOXANE, BENTONITE, PHENOXYETHANOL, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, METHYLPROPANEDIOL, TOCOPHERYL ACETATE, CAPRYLYL GLYCOL, CHLORPHENESIN, TRIETHOXYCAPRYLYLSILANE, SILANEDIOL SALICYLATE, OLIVE OIL DECYL ESTERS, TROMETHAMINE, LAURETH-12, CERAMIDE 3, CITRIC ACID, HYDRATED SILICA, LACTIC ACID, SQUALENE, CHLORELLA VULGARIS EXTRACT, SEA SALT, TOCOPHEROL. MAY CONTAIN: TITANIUM DIOXIDE, IRON OXIDES, MICA.
PRINCIPAL DISPLAY PANEL - 30 g Tube Box - CLARO 1 - BEIGE
ésika
PRO
INSTANT PERFECTION
3 EN 1
Face Refining Foundation
SPF 25
Net Wt. 1 oz e (30 g)

PRINCIPAL DISPLAY PANEL - 30 g Tube Box - CLARO 2 - BEIGE
ésika
PRO
INSTANT PERFECTION
3 EN 1
Face Refining Foundation
SPF 25
Net Wt. 1 oz e (30 g)

PRINCIPAL DISPLAY PANEL - 30 g Tube Box - MEDIO 1 - BEIGE
ésika
PRO
INSTANT PERFECTION
3 EN 1
Face Refining Foundation
SPF 25
Net Wt. 1 oz e (30 g)

PRINCIPAL DISPLAY PANEL - 30 g Tube Box - MEDIO 2 - BEIGE
ésika
PRO
INSTANT PERFECTION
3 EN 1
Face Refining Foundation
SPF 25
Net Wt. 1 oz e (30 g)

PRINCIPAL DISPLAY PANEL - 30 g Tube Box - MEDIO 3 - BEIGE
ésika
PRO
INSTANT PERFECTION
3 EN 1
Face Refining Foundation
SPF 25
Net Wt. 1 oz e (30 g)

PRINCIPAL DISPLAY PANEL - 30 g Tube Box - MEDIO 4 - BEIGE
ésika
PRO
INSTANT PERFECTION
3 EN 1
Face Refining Foundation
SPF 25
Net Wt. 1 oz e (30 g)

PRINCIPAL DISPLAY PANEL - 30 g Tube Box - MEDIO 5 - BEIGE
ésika
PRO
INSTANT PERFECTION
3 EN 1
Face Refining Foundation
SPF 25
Net Wt. 1 oz e (30 g)

PRINCIPAL DISPLAY PANEL - 3 g Tube Box - CLARO 1 - BEIGE
ésika
PRO
INSTANT
PERFECTION 3 EN 1
Face Refining
Foundation SPF 25
Net Wt. 0.10 oz. e (3 g)
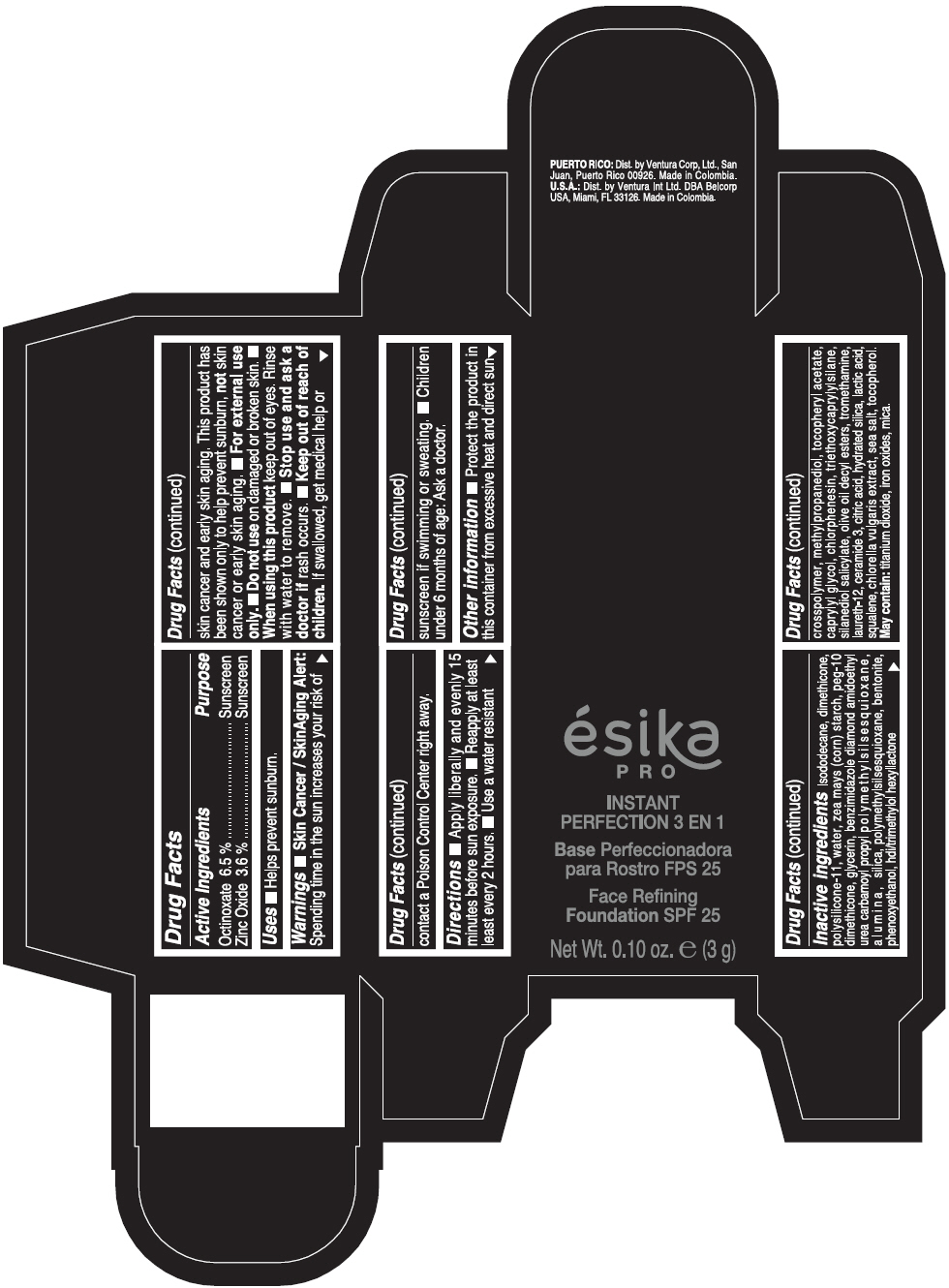
PRINCIPAL DISPLAY PANEL - 3 g Tube Box - CLARO 2 - BEIGE
ésika
PRO
INSTANT
PERFECTION 3 EN 1
Face Refining
Foundation SPF 25
Net Wt. 0.10 oz. e (3 g)
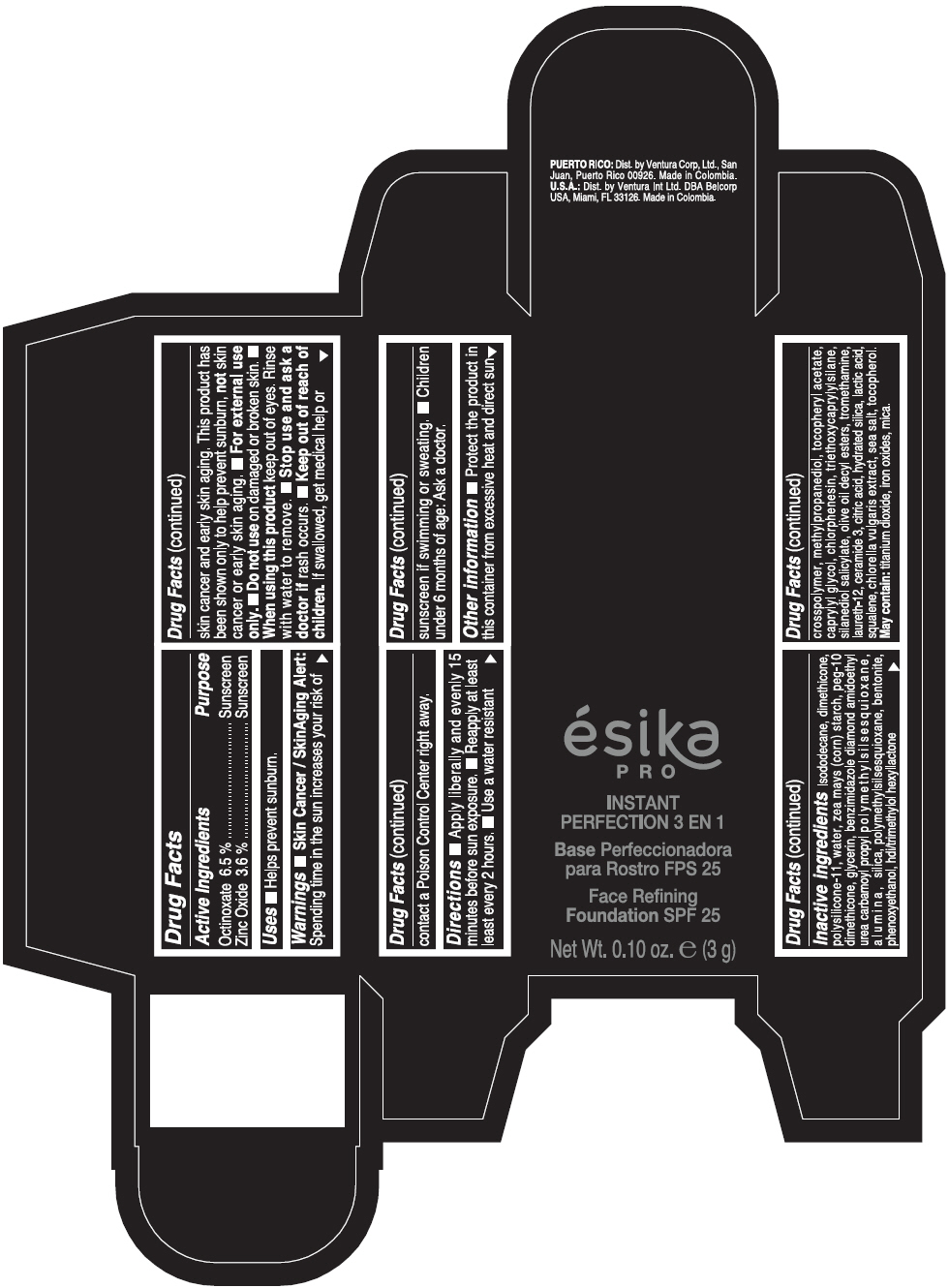
PRINCIPAL DISPLAY PANEL - 3 g Tube Box - MEDIO 1 - BEIGE
ésika
PRO
INSTANT
PERFECTION 3 EN 1
Face Refining
Foundation SPF 25
Net Wt. 0.10 oz. e (3 g)
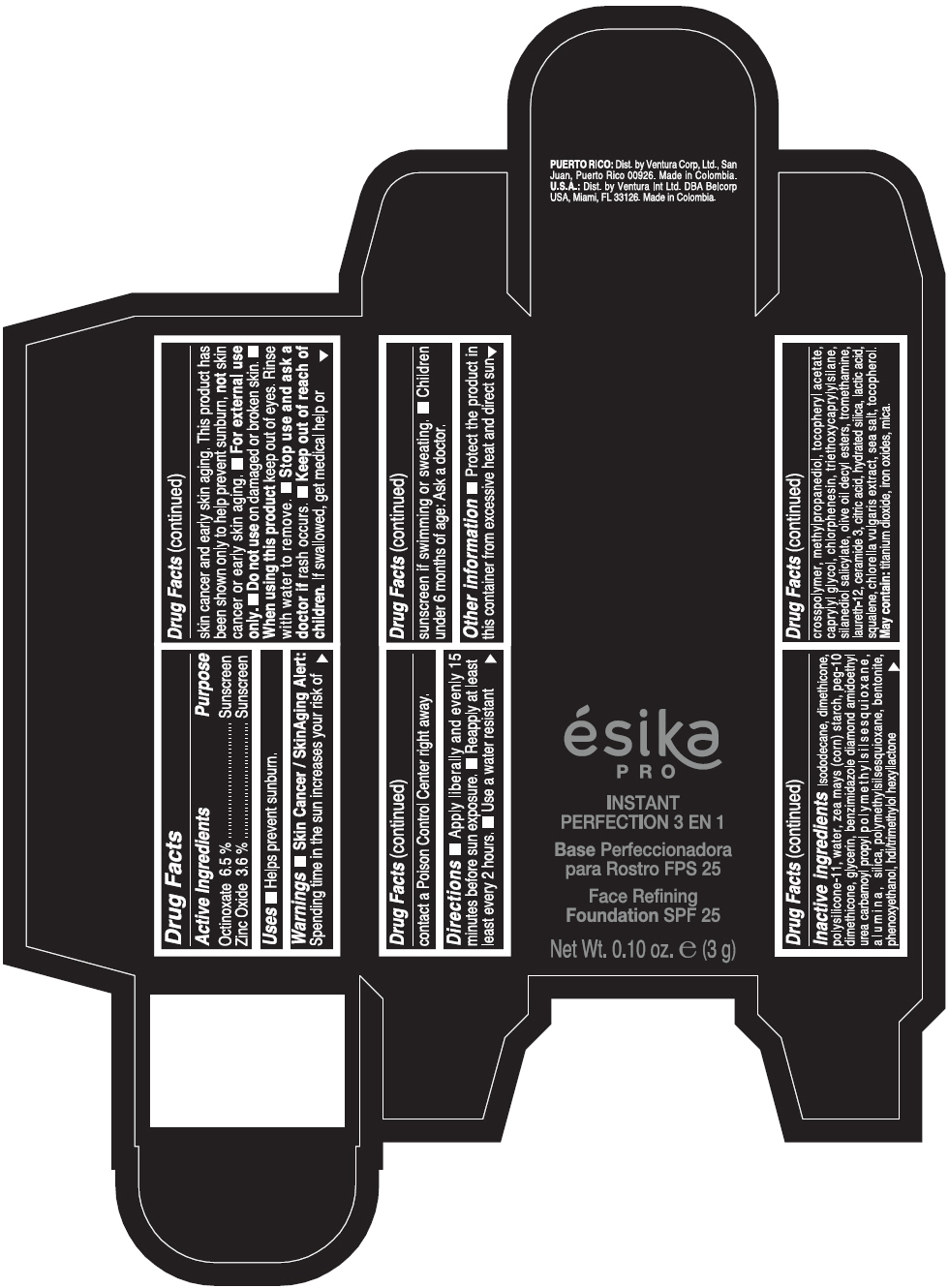
PRINCIPAL DISPLAY PANEL - 3 g Tube Box - MEDIO 2 - BEIGE
ésika
PRO
INSTANT
PERFECTION 3 EN 1
Face Refining
Foundation SPF 25
Net Wt. 0.10 oz. e (3 g)
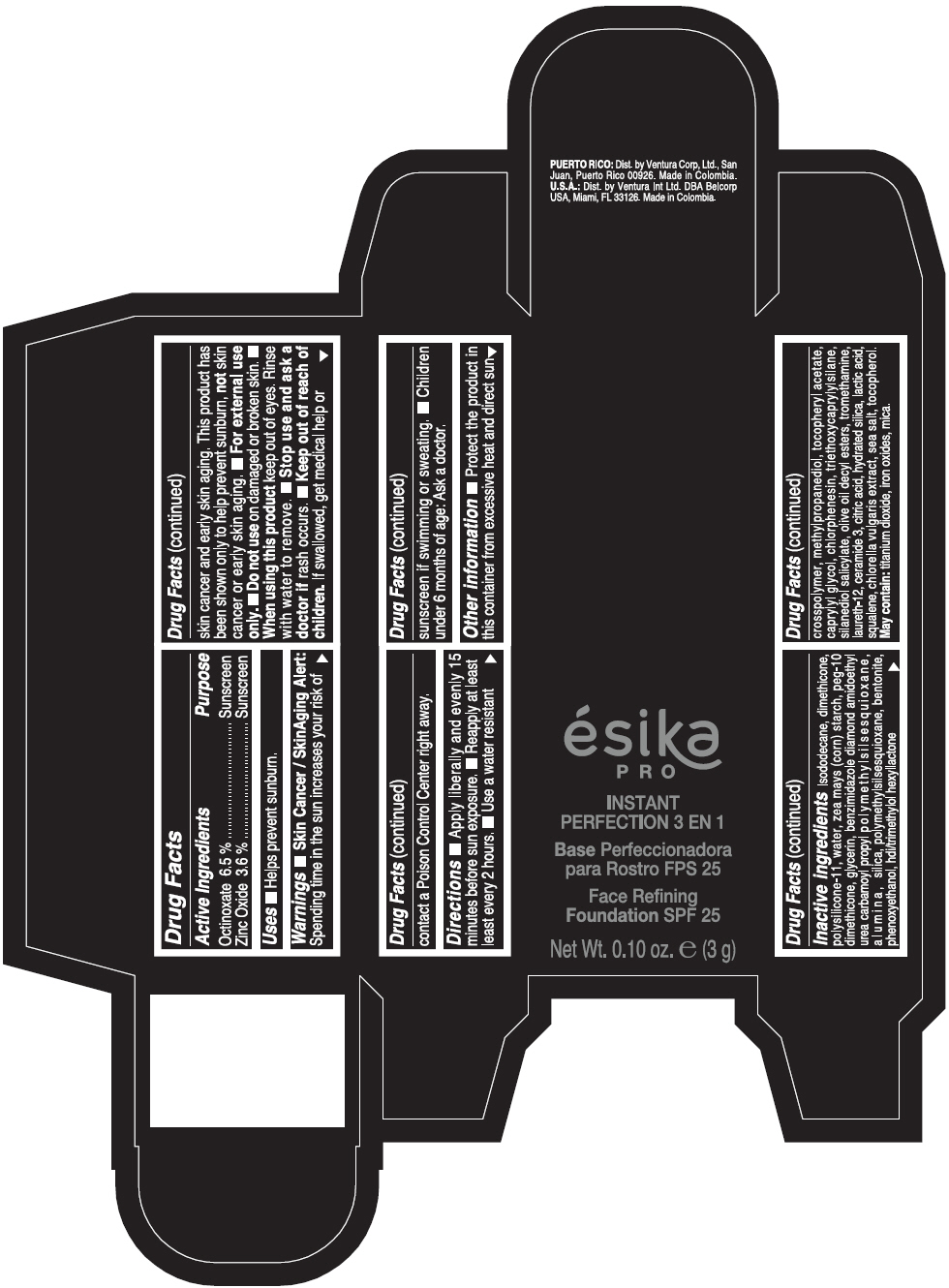
PRINCIPAL DISPLAY PANEL - 3 g Tube Box - MEDIO 3 - BEIGE
ésika
PRO
INSTANT
PERFECTION 3 EN 1
Face Refining
Foundation SPF 25
Net Wt. 0.10 oz. e (3 g)