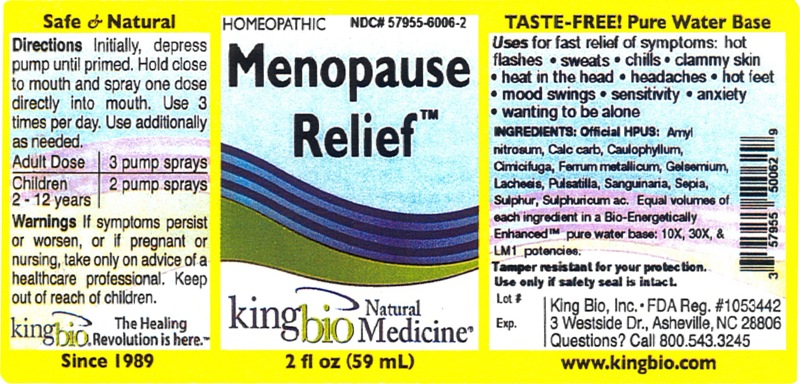
Active Ingredients
Official HPUS: Amyl nitrosum, Calcarea carbonica, Caulophyllum thalictroides, Cimicifuga racemosa, Ferrum metallicum, Gelsemium sempervirens, Lachesis mutus, Pulsatilla, Sanguinaria canadensis, Sepia, Sulphur, Sulphuricum acidum.
Reference image menopauserelief.jpg
Purpose
for fast relief of symptoms:
- hot flashes
- sweats
- chills
- clammy skin
- heat in the head
- headaches
- hot feet
- mood swings
- sensitivity
- anxiety
- wanting to be alone
Inactive Ingredient
Equal volumes of each ingredient in a Bio-Energetically Enhanced pure water base: 10X, 30X, and LM1 potencies.
Reference image menopauserelief.jpg
Warnings
If symptoms persist or worsen, or if pregnant or nursing, take only on advice of a healthcare professional. Keep out of reach of children.
Other: Tamper resistant for your protection. Use only if safety seal is intact.
Reference image menopauserelief.jpg
Indications and Usage
For fast relief of symptoms: hot flashes, sweats, chills, clammy skin, heat in the head, headaches, hot feet, mood swings, sensitivity, anxiety, wanting to be alone.
Reference image menopauserelief.jpg
Keep Out of Reach of Children
Warning: Keep out of reach of children.
Reference image menopauserelief.jpg