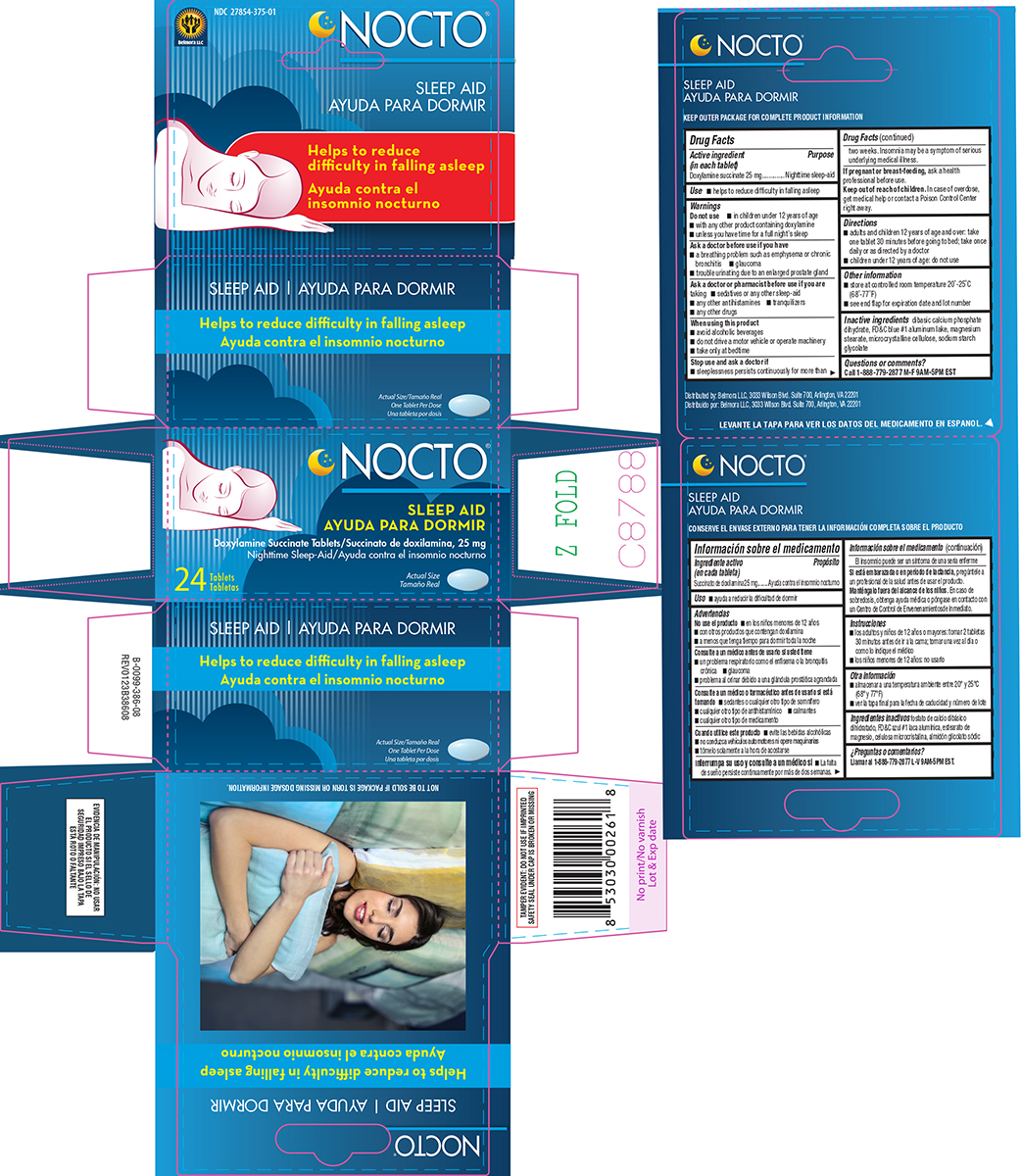
Warnings
Do not use
- in children under 12 years of age
- with any other product containing doxylamine
- unless you have time for a full night’s sleep
Ask a doctor before use if you have
- a breathing problem such as asthma, emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
taking
- sedatives or any other sleep-aid
- tranquilizers
- any other antihistamines
- any other drugs
When using this product
- avoid alcoholic beverages
- do not drive a motor vehicle or operate machinery
- take only at bedtime
Directions
- adults and children 12 years of age and over: take one tablet 30 minutes before going to bed; take once daily or as directed by a doctor
- children under 12 years of age: do not use
Other information
- store at controlled room temperature 20°-25°C (68°-77°F)
- see end flap for expiration date and lot number
Inactive ingredients
dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
Principal display panel
Belmora LLC
NDC 27854-375-01
NOCTO®
SLEEP AID
Doxylamine Succinate Tablets 25 mg
Nighttime Sleep-Aid
24 Tablets
Actual Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
NOT TO BE SOLD IF PACKAGE IS TORN OR MISSING DOSAGE INFORMATION.
Distributed by: Belmora LLC, 3033 Wilson Blvd. Suite 700, Arlington, VA 22201
Nocto 44-386