Forms and presentation
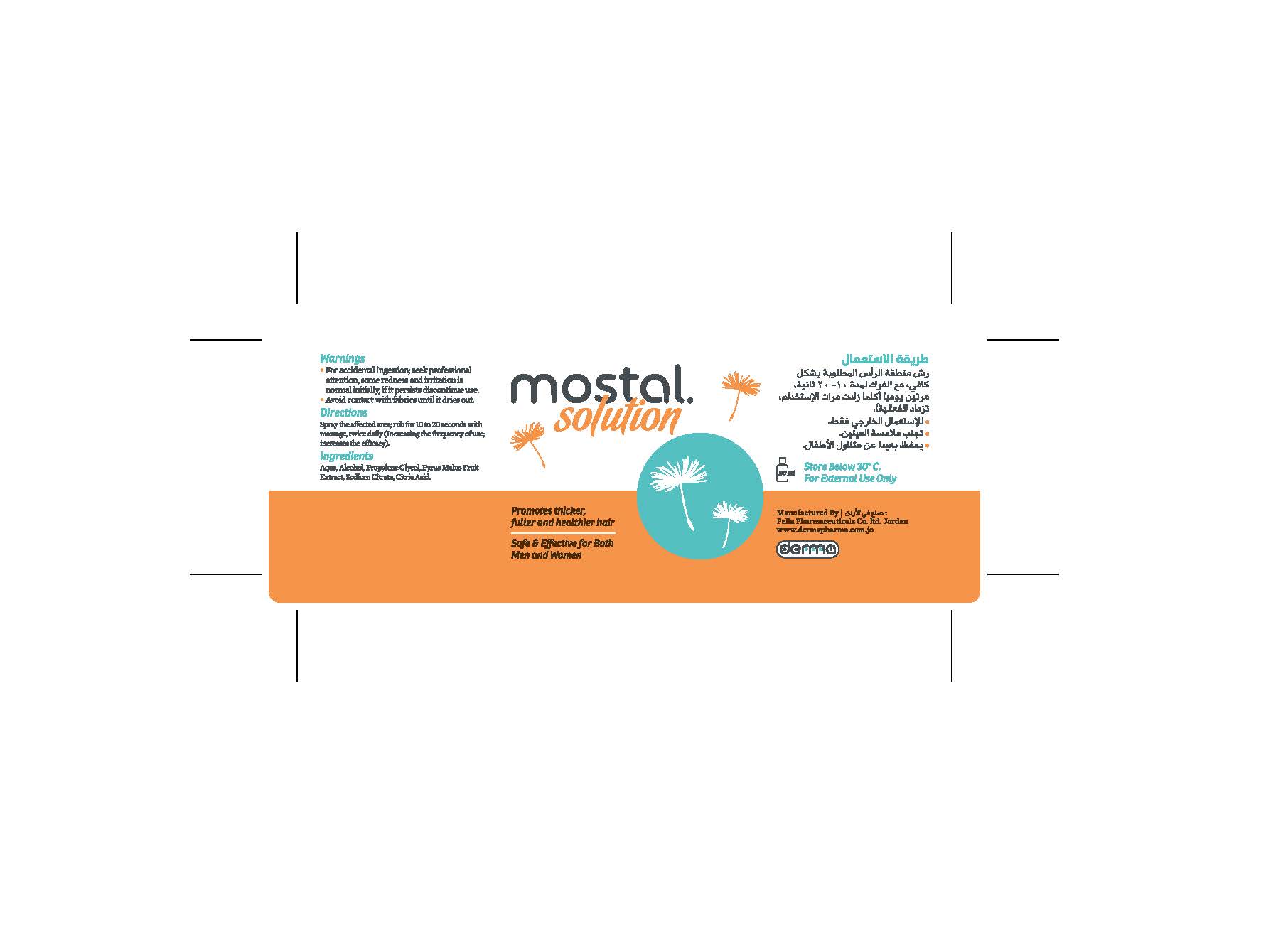
Topical Solution: Bottle of 50 ml.
Active Ingredient
Pyrus Malus (Apple) Fruit Extract (Procyanidine B2)
Inactive Ingredients
Aqua, Alcohol, Propylene Glycol, Sodium Citrate, Citric Acid.
Purpose
Hair growth enhancer
Properties
Mostal
® solution that helps to stop hair fall; improves hair growth and increases its density.
Indications
Mostal
® solution is recommended for men and women to stop hair fall; improves hair growth and increases its density.
Precautions
Keep out of reach of children
Warnings
- For external use only
- Avoid eye contact
- For accidental ingestion; seek professional attention, some redness and irritation is normal initially, if it persists discontinue use
- Avoid contact with fabrics until it dries out
Contraindications
Hypersensitivity to any of the components.
Side effects
Mostal
® solution has no known side effect, the use of it is without risk.
Dosage and administration
Spraying the solution on the scalp, the solution gently massaged into the scalp for 10 to 20 seconds, twice daily.
The increase in frequency of use, increases efficiency.
Storage conditions
Store at a temperature below 30 ° C.
Label
Secondary Package

Pella Pharmaceuticals Co. Ltd

