FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ACUVUE® Theravision™ with Ketotifen is a daily wear, daily disposable etafilcon A drug-eluting contact lens for prevention ocular itch due to allergic conjunctivitis and correction of refractive ametropia (myopia and hyperopia) in aphakic and/or phakic patients who do not have red eye(s), are suitable for contact lens wear and do not have more than 1 D of astigmatism.
The lens contains an H1 histamine receptor antagonist for the prevention of ocular itch due to allergic conjunctivitis. The prevention of itch has been demonstrated to last through 12 hours in clinical trials; however, the lens may be worn for longer than 12 hours in a single day.
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
One ACUVUE® Theravision™ with Ketotifen should be inserted per eye per day. Discard lens after a single day's use. ACUVUE® Theravision™ with Ketotifen may be worn beyond twelve hours for vision correction. Lenses should be removed prior to sleeping.
The maximum daily wearing time should be determined by the Eye Care Professional based upon the patient's individual response to contact lenses. Patients tend to over wear the lenses initially. The Eye Care Professional should emphasize the importance of adhering to the initial maximum daily wearing time. Regular checkups, as determined by the Eye Care Professional, are also extremely important.
Eye drops containing benzalkonium chloride should not be used simultaneously with this product. Patients should wait 10 minutes after applying eye drop with benzalkonium chloride before inserting or reinserting lenses.
If the lens sticks (stops moving), the patient should be instructed to remove the lens. A few drops of non-preserved sterile saline solution may be applied directly to the eye to assist with removal. If non-movement of the lens continues after a few minutes, the patient should immediately consult their Eye Care Professional.
Do not use contact lens cleaning and disinfectant solutions with ACUVUE® Theravision™ with Ketotifen.
2.2 Contact Lens Fitting Information
See the ACUVUE® Theravision™ with Ketotifen Fitting Instruction Guide for professional fitting instructions and information.
3 DOSAGE FORMS AND STRENGTHS
Daily disposable etafilcon A drug-eluting contact lens with ketotifen (19 mcg per lens).
4 CONTRAINDICATIONS
4.1 Ocular Hyperemia
ACUVUE® Theravision™ with Ketotifen is contraindicated in patients with ocular hyperemia and should not be inserted into red or irritated eye(s). Remove ACUVUE Theravision with Ketotifen immediately if eye(s) becomes red or irritated.
5 WARNINGS AND PRECAUTIONS
5.1 Corneal Ulcers
ACUVUE Theravision with Ketotifen should not be inserted if the patient has corneal epithelial breakdown, corneal ulceration, dry eye disease, injury or abnormality that affects the eyelids or anterior segment of the eye. Contact lens wear may worsen these conditions and lead to sight threatening complications. If a patient experiences eye discomfort, excessive tearing, vision changes, or redness of the eye, the patient should be instructed to immediately remove the lenses, and promptly contact the Eye Care Professional.
Studies have shown that when daily wear users wear their lenses overnight, the risk of ulcerative keratitis is greater than among those who do not wear them overnight. Contact lens wearers who are smokers have a higher incidence of corneal ulcers than nonsmokers.
5.2 Contact Lens-Related Complications
ACUVUE Theravision with Ketotifen should not be used to treat or prevent lens-related symptoms including irritation, discomfort or redness. ACUVUE Theravision with Ketotifen insertion could result in serious injury if a patient is experiencing these symptoms. Patients should be cautioned that proper use and care of contact lenses and lens care products are essential for the safe use of these products. If patients experience symptoms of contact lens intolerance or keratitis, the lens(es) should be IMMEDIATELY REMOVED.
5.3 Chemical Exposures
If chemicals of any kind (household products, gardening solutions, laboratory chemicals, etc.) are splashed into the eyes, the patient should: FLUSH EYES IMMEDIATELY WITH WATER AND IMMEDIATELY CONTACT THE EYE CARE PROFESSIONAL OR VISIT A HOSPITAL EMERGENCY ROOM WITHOUT DELAY.
5.4 Acanthamoeba Keratitis
ACUVUE Theravision with Ketotifen should not be worn while swimming or in hot tubs. The lens should not be rinsed in water from the tap. Tap water contains many impurities that can contaminate or damage the lenses and may lead to eye infection or injury. Contact lens wear in these settings increases the risk of sight threatening eye infections from microorganisms.
5.5 Backup Spectacles
Eye Care Professionals should instruct the patient to always have a functional pair of spectacles with a current prescription available to use if the patient becomes unable to wear contact lenses, or in circumstances where contact lens wear is not advised.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Ocular Hyperemia [see Contraindications (4.1)]
- Corneal Hypoesthesia [see Contraindications (4.2)]
- Corneal Infections [see Contraindications (4.3)]
- Corneal Ulcers [see Warnings and Precautions (5.1)]
- Contact Lens-Related Complications [see Warning and Precautions (5.2)]
- Chemical Exposure [see Warnings and Precautions (5.3)]
- Acanthamoeba Keratitis [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in practice.
The most commonly observed adverse reactions in clinical studies, occurring in ≥1% of ACUVUE® Theravision™ with Ketotifen treated eyes, were eye irritation, eye pain, and instillation site irritation.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of ACUVUE® Theravison™ with Ketotifen administration in pregnant women to inform a drug-associated risk. ACUVUE® Theravison™ with Ketotifen is not absorbed systemically following ocular administration, and maternal use is not expected to result in fetal exposure to the drug. Oral administration of ketotifen fumarate to pregnant rats or rabbits did not produce teratogenicity at clinically relevant doses [see Data].
Data
Human Data
There are no human data that establish the presence or absence of drug-associated risk with the use of ACUVUE® Theravision™ with Ketotifen by pregnant women.
Animal Data
Oral administration of 45 mg/kg/day ketotifen to pregnant rabbits resulted in an increased incidence of retarded ossification of the sternebrae. This dose (normalized to body surface area) was approximately 23,000 times higher than the daily maximum recommended human ophthalmic dose (MRHOD) of 0.038 mg.
No adverse embryofetal effects were observed in rats or rabbits orally administered 100 mg/kg/day and 15 mg/kg/day, respectively, during organogenesis. These doses (normalized to body surface area) were approximately 26,000 and 7,700 times higher than the MRHOD, respectively.
An oral dose of 50 mg/kg/day ketotifen (approximately 13,000 times higher than MRHOD) administered to rats from Day 15 of pregnancy until Day 21 postpartum produced maternal toxicity, a slight increase in postnatal mortality and a slight decrease in body weight gain in offspring during the first four days post-partum.
8.2 Lactation
Risk Summary
There is no information regarding the presence or absence of ketotifen fumarate or its metabolites in human milk following use of ACUVUE® Theravision™ with Ketotifen, or on the breastfed infants and milk production. Ketotifen fumarate has been identified in breast milk in rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in breast milk.
The development and health benefits of breastfeeding should be considered along with the mother's clinical need for ACUVUE® Theravision™ with Ketotifen and any potential adverse effects on the breast-fed child from ketotifen fumarate.
11 DESCRIPTION
ACUVUE® Theravision™ with Ketotifen is a sterile, soft (hydrophilic), spherical daily wear, daily disposable etafilcon A drug-eluting contact lens containing ketotifen, an H1 receptor antagonist, for topical administration to the eyes.
Ketotifen fumarate is a white to brownish-yellow, fine crystalline powder. The buffered packaging solution of ACUVUE® Theravision™ with Ketotifen has a pH range of 6.6 to 7.3 and an osmolality of not more than 460 mOsm/Kg. This product does not contain an antimicrobial preservative.
Contains:
Active: ketotifen 19 mcg equivalent to 43 mcg ketotifen fumarate per lens
Inactives: boric acid, calcium hydroxide, pentetic acid, sodium chloride, sodium borate, and water.
Chemical Name: 4,9-Dihydro-4-(1-methyl-4-piperidylidene)-10H-benzo[4,5]cyclohepta[1,2-b] thiophen-10-one fumarate (1:1).
Structural Formula:
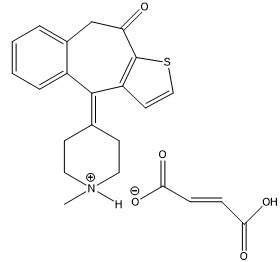
| Molecular formula of C19H19NOS∙C4H4O4 (C19H19NOS as free base) |
| Molecular weight of 425.50 g/mol (309.43 g/mol as the free base) |
The contact lens material (etafilcon A) is a copolymer of 2-hydroxyethyl methacrylate and methacrylic acid cross-linked with 1, 1, 1-trimethylol propane trimethacrylate and ethylene glycol dimethacrylate. ACUVUE® Theravision™ with Ketotifen contact lenses are tinted blue using blue 2-hydroxyethyl methacrylate to make the lenses more visible for handling.
Physical/Optical Properties of the Lens:
| Refractive Index: | 1.40 |
| Visible Light Transmission: | 90% minimum |
| Ultraviolet Light Tranmission: (316 nm to 380 nm) UVA | 30% maximum |
| Ultraviolet Light Tranmission: (280 nm to 315 nm) UVB | 5% maximum |
| Surface Character: | Hydrophilic |
| Water Content: | 59% |
| Oxygen Permeability: | 21.4 × 10-11(cm2/sec) (mL O2/mL × mmHg) at 35°C (boundary corrected, edge corrected) |
Dimensional Properties of the Lens:
| Diameter: | 14.2 mm |
| Center Thickness: | Minus Lens - varies with power (e.g. -4.00D: 0.084 mm) |
| Plus Lens - varies with power (e.g. +4.00D: 0.190 mm) | |
| Base Curve: | 8.5 mm and 9.0 mm |
| Power: | -0.50D to -10.00D (in 0.25D increments) |
| -10.50D to -12.00D (in 0.50D increments) | |
| +0.50D to +6.00D (in 0.25D increments) |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Device Component
In its hydrated state, the lens when placed on the cornea, acts as a refracting medium to focus light rays on the retina to correct refractive ametropia for as long as the lens is worn (up to 24 hours while awake).
Drug Component
Ketotifen fumarate, a benzocycloheptathiophene derivative, is a H1 receptor antagonist that stabilizes mast cells and prevents eosinophil accumulation. This action prevents the onset of ocular allergic itch allowing for continued lens wear during episodes of allergen exposure and has been demonstrated to last through 12 hours.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
Ketotifen fumarate was determined to be non-mutagenic in a battery of in vitro and in vivo mutagenicity assays including: Ames test, in vitro chromosomal aberration test with V79 Chinese hamster cells, in vivo micronucleus assay in mouse, and mouse dominant lethal test. In addition, extracts of etafilcon A with ketotifen (19 mcg/lens) prepared in 0.9% sodium chloride or dimethyl sulfoxide were shown to be non-mutagenic in the Ames test.
Impairment of Fertility
Treatment of male rats with oral doses of ketotifen >10 mg/kg/day (approximately 2,600 times the MRHOD) for 70 days prior to mating resulted in mortality and a decrease in fertility. Treatment with ketotifen did not impair fertility in female rats receiving up to 50 mg/kg/day of ketotifen orally (approximately 13,000 times the MRHOD) for 15 days prior to mating.
14 CLINICAL STUDIES
The safety and efficacy of ACUVUE® Theravision™ with Ketotifen was assessed in two double-masked, randomized, placebo-controlled clinical conjunctival allergen challenge (CAC) studies (CR-4483 and CR-4484) and two 12-week safety studies (CR-4490 and CR-4539). Patients in these studies had allergic conjunctivitis induced by an ocular allergen challenge and refractive ametropia (myopia and hyperopia) suitable for contact lens wear without more than 1.00 D of astigmatism. A total of 244 patients (488 eyes) were evaluate in the CAC studies. A total of 491 subjects were exposed to ACUVUE® Theravision™ with Ketotifen over a period of 12 weeks in the safety studies. These four studies demonstrated that ACUVUE® Theravision™ with Ketotifen was safe and more effective than placebo (1•DAY ACUVUE®) in preventing ocular itching in patients with allergic conjunctivitis. ACUVUE® Theravision™ with Ketotifen reduced ocular itching within 3 minutes and the response was sustained for up to 12 hours after lens insertion. Visual acuity was comparable between ACUVUE® Theravision™ with Ketotifen and 1•DAY ACUVUE®.
Not all refractive powers, design configurations, or lens parameters available were tested in clinical investigation of the lenses. Therefore, when selecting an appropriate lens design, the Eye Care Professional should consider all characteristics of the lens that can affect lens performance and ocular health, including oxygen permeability, wettability, central and peripheral thickness and optic zone diameter. The potential impact of these factors on the patient's ocular health should be carefully weighed against the patient's need for refractive correction and prevention of itching associated with allergic conjunctivitis; therefore, the continuing ocular health of the patient and lens performance on the eye should be carefully monitored by the prescribing Eye Care Professional.
16 HOW SUPPLIED/STORAGE AND HANDLING
ACUVUE® Theravision™ with Ketotifen (etafilcon A drug-eluting contact lens with ketotifen) is a daily disposable contact lens, 19 mcg ketotifen per lens is supplied in a plastic blister bowl and a foil laminated lidstock containing a buffered ketotifen solution. The plastic package is marked with base curve, lens diameter, diopter (lens power), date of manufacture, expiration date and lot number.
ACUVUE® Theravision™ with Ketotifen is supplied in a 30-count carton containing 30 foil sealed blister packages containing etafilcon A contact lens with 19 mcg ketotifen (NDC 60123-019-30).
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Fitting
See the ACUVUE® Theravision™ with Ketotifen FITTING INSTRUCTION GUIDE for professional fitting instructions and information.
Wearing Schedule (Daily Wear)
Advise patients that ACUVUE® Theravision™ with Ketotifen should not be used to treat red eye(s). Remove lens(es) immediately if the eye(s) become red or irritated.
A single ACUVUE® Theravision™ with Ketotifen should be worn in each eye per day and should be discarded upon removal each day. The lens should not be worn beyond the period recommended by an eye care professional.
Advise patients that ACUVUE® Theravision™ with Ketotifen should be removed prior to sleep.
Lens Handling and Care
Advise patients to not use if the ACUVUE® Theravision™ with Ketotifen lens sterile blister package is opened or damaged.
Advise patients to always wash and rinse hands before handling lenses.
Advise patients to avoid contaminating hands or lenses with cosmetics, lotions, soaps, creams, deodorants or sprays. Instruct patients to put in lenses prior to applying makeup.
Advise patients not to touch contact lenses if hands have any foreign materials as microscopic scratches of the lenses may occur, which can cause distorted vision and/or injury to the eye.
Instruct patients to examine the lens after opening to be sure that it is a single, moist, clean lens that is free of any nicks or tears. If the lens appears damaged, patients should be instructed NOT to use it.
Instruct patients not to use contact lens cleaning and disinfectant solutions with ACUVUE® Theravision™ with Ketotifen. Discard lens after a single day's use.
Advise patients that ACUVUE® Theravision™ with Ketotifen should not be worn while swimming or in hot tubs. Contact lens wear in these settings increases the risk of sight threatening eye infections from microorganisms.
If the lens sticks (stops moving), the patient should be instructed to remove the lens. A few drops of non-preserved sterile saline solution may be applied directly to the eye to assist with removal. If non-movement of the lens continues after a few minutes, the patient should immediately consult their Eye Care Professional.
Patients should be advised to notify the employer of being a contact lens wearer. Some jobs may require the use of eye protection equipment or may require that the patient not wear contact lenses.
Advise patients to avoid all harmful or irritating vapors and fumes while wearing lenses. If aerosol products, such as hair spray, are used while wearing lenses, exercise caution and keep eyes closed until the spray has settled.
Instruct patients to never use tweezers, fingernails, or other tools to remove lenses from the lens container. Instead, patients should remove ACUVUE® Theravision™ with Ketotifen from the blister container packing solution using their fingertips. Patients should not touch the lens with fingernails.
The patients should be advised to never rinse the lenses in water from the tap. Tap water contains many impurities that can contaminate or damage the lenses and may lead to eye infection or injury.
Advise patients to always dispose of lenses when removed and have spare non-medicated lenses or spectacles available.
Concomitant Use of Other Medications
Advise patients to consult with their eyecare professional before using any medicine in the eyes. Advise patients that eye drops containing benzalkonium chloride should not be used simultaneously with ACUVUE® Theravision™ with Ketotifen. Instruct patients to wait 10 minutes after eye drop application before inserting lenses.
Daily self-examination
Instruct the patient to immediately remove the lens if any eye discomfort, eye pain, eye redness or decreased vision are experienced. If the symptoms stop after lens removal, the patient should discard the lens(es) and replace with new non-medicated lens(es). ACUVUE® Theravision™ with Ketotifen should not be worn for the remainder of the day. If the problem continue after inserting a new non-medicated lens, the patient should remove the lens and IMMEDIATELY CONSULT THEIR EYE CARE PROFESSIONAL.
Emergencies
Advise the patient that if chemicals of any kind (household products, gardening solutions, laboratory chemicals, etc.) are splashed into the eyes, the patient should: FLUSH EYES IMMEDIATELY WITH TAP WATER AND IMMEDIATELY CONTACT THE EYE CARE PROFESSIONAL OR VISIT A HOSPITAL EMERGENCY ROOM WITHOUT DELAY.
Symbols Key
The following symbols may appear on the label or packaging:
| SYMBOL | DEFINITION |
|---|---|
 | Caution, Consult Instructions for Use |
 | Manufacturer |
 | Date of Manufacture |
 | Use by Date (expiration date) |
 | Batch Code |
 | Sterilized Using Steam Heat |
 | Do Not Re-Use (Single Use) |
 | Do Not Use if Package is Damaged |
 | Store Away from Direct Sunlight |
 | Indicates a Single Sterile Barrier System |
 | Authorized Representative in the European Community |
 | Medical Device in the European Community |
 | Fee Paid for Waste Management |
 | CE-mark and Identification Number of Notified Body |
 | Contains Hazardous Substances |
 | Contains a Medicinal Substance |
 | CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed practitioner |
| CONTACT LENS | Contact Lens |
 | Lens Orientation Correct |
 | Lens Orientation Incorrect (Lens Inside Out) |
 | Package Opening Icon (Blister) |
| DIA | Diameter |
| BC | Base Curve |
| D | Diopter (Lens Power) |
Distributed by:
Johnson & Johnson Vision Care, Inc.
7500 Centurion Parkway
Jacksonville, Florida 32256, USA
www.acuvue.com
©Johnson & Johnson Vision Care, Inc. 2021
In Canada: Johnson & Johnson Vision Care, division of Johnson & Johnson, Inc.
In USA: Johnson & Johnson Vision Care, Inc.
Printed in USA
Revision date: 2/2022
Revision number: XXXXX
ACUVUE® Theravision™ is a trademark of Johnson & Johnson Vision Care, Inc.
ACUVUE®
Theravision™ with Ketotifen
(etafilcon A lens with ketotifen)
For Eye Itch
for Daily Disposable Wear
FITTING INSTRUCTION GUIDE
Rx Only
CAUTION: US Federal law restricts this device to sale by or on the order of a licensed practitioner
PRODUCT DESCRIPTION
For full prescribing information see the ACUVUE® Theravision™ with Ketotifen Package Insert.
ACUVUE® Theravision™ with Ketotifen is a sterile, soft (hydrophilic), spherical etafilcon A drug-eluting contact lens containing ketotifen, an H1 receptor antagonist, for topical administration to the eyes.
Ketotifen fumarate is a white to brownish-yellow, fine crystalline powder with a molecular formula of C19H19NOS-C4H4O4 and formula weight of 425.50 g/mol. The buffered packaging solution of ACUVUE® Theravision™ with Ketotifen has a pH of 6.6 to 7.3 and an osmolality of 380 to 460 mOsm/Kg.
Contains:
- Active: ketotifen 19 mcg per lens
- Inactives: boric acid, calcium hydroxide, pentetic acid, sodium chloride, sodium borate, and water.
Chemical Name: 10H-Benzo[4,5]cyclohepta[1,2-b]thiophen-10-one, 4,9 dihydro-4-(1-methyl-4-piperidinylidene)-, hydrogen fumarate.
Structural Formula:
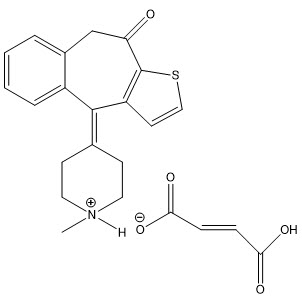
.This product does not contain an antimicrobial preservative.
The contact lens material (etafilcon A) is a copolymer of 2-hydroxyethyl methacrylate and methacrylic acid cross-linked with 1, 1, 1-trimethylol propane trimethacrylate and ethylene glycol dimethacrylate.
ACUVUE® Theravision™ with Ketotifen contact lenses are tinted blue using blue 2-hydroxyethyl methacrylate to make the lenses more visible for handling.
Physical/Optical Properties of the Lens:
| 0.98 – 1.12 |
| 1.40 |
| 90% minimum |
| Hydrophilic |
| 59% |
| |
| METHOD |
Dosage Forms and Strengths:
etafilcon A drug-eluting contact lens with ketotifen
(19 mcgper lens).
How Supplied:
Each ACUVUE® Theravision™ with Ketotifen (etafilcon A drug-eluting contact lens with ketotifen) lens is supplied in a foil-sealed plastic package containing a buffered ketotifen solution. The plastic package is marked with base curve, diopter power, diameter, lot number and expiration date.
AVAILABLE LENS PARAMETERS
The ACUVUE® Theravision™ with Ketotifen Brand Contact Lenses are hemispherical shells of the following dimensions:
| Diameter (DIA): | 14.2 mm |
| Center Thickness: | Minus Lens - varies with power (e.g. -4.00D: 0.084 mm) Plus Lens - varies with power (e.g. +4.00D: 0.190 mm) |
| Base Curve (BC): | 8.5 mm and 9.0 mm |
| Powers (D): | -0.50D to -10.00D (in 0.25D increments) -10.50D to -12.00D (in 0.50D increments) +0.50D to +6.00D (in 0.25D increments) |
ACTIONS
See CLINICAL PHARMACOLOGY, Mechanism of Action section of the ACUVUE® Theravision™ with Ketotifen Package Insert.
INDICATIONS AND USES
See INDICATIONS AND USAGE section of the ACUVUE® Theravision™ with Ketotifen Package Insert.
CONTRAINDICATIONS (REASONS NOT TO USE)
See CONTRAINDICATIONS section of the ACUVUE® Theravision™ with Ketotifen Package Insert.
WARNINGS
See WARNINGS AND PRECAUTIONS section of the ACUVUE® Theravision™ with Ketotifen Package Insert.
PRECAUTIONS
See WARNINGS AND PRECAUTIONS section of the ACUVUE® Theravision™ with Ketotifen Package Insert.
ADVERSE REACTIONS
See ADVERSE REACTIONS section of the ACUVUE® Theravision™ with Ketotifen Package Insert.
FITTING
GENERAL FITTING GUIDELINES
Patient Selection
You should first assess the patient's needs to ensure that the patient is an appropriate candidate for ACUVUE® Theravision™ with Ketotifen. ACUVUE® Theravision™ with Ketotifen, like other soft contact lenses, will require the appropriate and usual physiological and diagnostic assessments necessary to ensure proper patient selection.
Pre-Fitting Examination
A pre-fitting patient history and examination are necessary to:
- Determine whether a patient is a suitable candidate for ACUVUE® Theravision™ with Ketotifen Contact Lens (consider allergies, patient hygiene and mental and physical state), and
- Take ocular measurements for the initial contact lens parameter selection, and
- Collect and record baseline clinical information to which post-fitting examination results can be compared.
A pre-fitting examination may include a determination of optimal distance and near spectacle correction and corneal curvature measurements. The near correction should be determined at the midpoint of the patient's habitual reading distance. When more than one power provides optimal reading performance, prescribe the least plus (most minus) of the powers that meet the patient's requirements.
Lens Selection
A. Initial Power Determination
A spectacle refraction should be performed to establish the patient's baseline refractive status and to guide in the selection of the appropriate lens power. Remember to compensate for vertex distance if the refraction is greater than ±4.00 D.
B. Base Curve Selection (Trial Lens Fitting)
An 8.5mm/14.2mm ACUVUE® Theravision™ with Ketotifen Contact Lens should be the initial lens of choice for myopic patients regardless of keratometry readings. However, corneal curvature measurements should be performed to establish the patient's baseline ocular status.
An 8.5mm/14.2mm ACUVUE® Theravision™ with Ketotifen Contact Lens should be placed on each of the patient's eyes and evaluated after the patient has adjusted to the lenses. A properly fit lens will center and completely cover the cornea (i.e., no limbal exposure), have sufficient movement with the blink to provide tear exchange under the contact lens and be comfortable. The lens should move freely when manipulated digitally with the lower lid, and then return to its properly centered position when released. If resistance is encountered when pushing the lens up, the lens is fitting tightly and should not be dispensed to the patient.
A steep fitting lens may exhibit one or more of the following characteristics: insufficient movement with the blink, conjunctival indentation and resistance when pushing the lens up digitally with the lower lid. If the 8.5mm/14.2mm ACUVUE® Theravision™ with Ketotifen Contact Lens is judged to be steep fitting, it should not be dispensed to the patient.
A flat fitting lens may exhibit one or more of the following characteristics: decentration, incomplete corneal coverage (i.e., limbal exposure), excessive movement with the blink or edge standoff. If the 8.5mm/14.2mm ACUVUE® Theravision™ with Ketotifen Contact Lens is judged to be flat fitting, it should not be dispensed to the patient.
If the initial ACUVUE® Theravision™ with Ketotifen Contact Lens base curve is judged to be flat or steep fitting, the alternate base curve, if available, should be considered.
C. Final Lens Power
A spherical over-refraction should be performed to determine the final lens power after the lens fit is judged acceptable. The spherical over-refraction should be combined with the trial lens power to determine the final lens prescription.
- Example 1:
- Diagnostic lens: -2.00 D
Spherical over-refraction -0.25 D
Final lens power: -2.25 D
- Example 2:
- Diagnostic lens: -2.00 D
Spherical over-refraction +0.25 D
Final lens power: -1.75 D
MONOVISION FITTING GUIDELINES
Patient Selection
A. Monovision Needs Assessment
For a good prognosis the patient should have adequately corrected distance and near visual acuity in each eye. The amblyopic patient or the patient with significant astigmatism (greater than 1.00D) in one eye may not be a good candidate for monovision correction with the ACUVUE® Theravision™ with Ketotifen Contact Lens.
Occupational and environmental visual demands should be considered. If the patient requires critical vision (visual acuity and stereopsis), it should be determined by trial whether this patient can function adequately with monovision correction.
Monovision contact lens wear may not be optimal for such activities as:
- (1)
- Visually demanding situations such as operating potentially dangerous machinery or performing other potentially hazardous activities: and
- (2)
- Driving automobiles (e.g., driving at night). Patients who cannot pass their state drivers license requirements with monovision correction should be advised to not drive with this correction, OR may require that additional over-correction be prescribed
B. Patient Education
All patients do not function equally well with monovision correction. Patients may not perform as well for certain tasks with this correction as they have with bifocal reading glasses. Each patient should understand that monovision, as well as other presbyopic alternatives, can create a vision compromise that may reduce visual acuity and depth perception for distance and near tasks. During the fitting process it is necessary for the patient to realize the disadvantages as well as the advantages of clear near vision and straight ahead and upward gaze that monovision contact lenses provide.
Eye Selection
Generally, the non-dominant eye is corrected for near vision. The following two methods for eye dominance can be used.
A. Ocular Preference Determination Methods
- Method 1 Determine which eye is the "sighting eye". Have the patient point to an object at the far end of the room. Cover one eye. If the patient is still pointing directly at the object, the eye being used is the dominant (sighting) eye.
- Method 2 Determine which eye will accept the added power with the least reduction in vision. Place a hand-held trial lens equal to the spectacle near ADD in front of one eye and then the other while the distance refractive error correction is in place for both eyes. Determine whether the patient functions best with the near ADD lens over the right or left eye.
Other methods include the refractive error method and the visual demands method.
B. Refractive Error Method
For anisometropic correction, it is generally best to fit the more hyperopic (less myopic) eye for distance and the more myopic (less hyperopic) eye for near.
C. Visual Demands Method
Consider the patient's occupation during the eye selection process to determine the critical vision requirements. If a patient's gaze for near tasks is usually in one direction, correct the eye on that side for near.
- Example:
- A writer who places copy to the left side of the desk will function best with the near lens on the left eye.
SPECIAL FITTING CHARACTERISTICS
Near ADD Determination
Always prescribe the lens power for the near eye that provides optimal near acuity at the midpoint of the patient's habitual reading distance. However, when more than one power provides optimal reading performance, prescribe the least plus (most minus) of the powers.
Trial Lens Fitting
A trial fitting is performed in the office to allow the patient to experience monovision correction. Lenses are fit according to the General Fitting Guidelines for base curve selection described in this guide.
Case history and standard clinical evaluation procedure should be used to determine the prognosis. Determine the distance correction and the near correction. Next determine the near ADD. With trial lenses of the proper power in place, observe the reaction to this mode of correction.
Allow the lenses to settle for about 20 minutes with the correct power lenses in place. Walk across the room and have the patient look at you. Assess the patient's reaction to distance vision under these circumstances. Then have the patient look at familiar near objects such as a watch face or fingernails. Again assess the reaction. As the patient continues to look around the room at both near and distance objects, observe the reactions. Only after these vision tests are completed should the patient be asked to read print. Evaluate the patient's reaction to large print (e.g., typewritten copy) at first and then graduate to newsprint and finally smaller type sizes.
After the patient's performance, under the above conditions, is completed, tests of visual acuity and reading ability under conditions of moderately dim illumination should be attempted.
An initial unfavorable response in the office, while indicative of a guarded prognosis, should not immediately rule out a more extensive trial under the usual conditions in which a patient functions.
Adaptation
Visually demanding situations should be avoided during the initial wearing period. A patient may at first experience some mild blurred vision, dizziness, headaches and a feeling of slight imbalance. You should explain the adaptational symptoms to the patient. These symptoms may last for a brief minute or for several weeks. The longer these symptoms persist, the poorer the prognosis for successful adaptation.
To help in the adaptation process, the patient can be advised to first use the lenses in a comfortable familiar environment such as in the home.
Some patients feel that automobile driving performances may not be optimal during the adaptation process. This is particularly true when driving at night. Before driving a motor vehicle, it may be recommended that the patient be a passenger first to make sure that their vision is satisfactory for operating an automobile. During the first several weeks of wear (when adaptation is occurring), it may be advisable for the patient to only drive during optimal driving conditions. After adaptation and success with these activities, the patient should be able to drive under other conditions with caution.
Other Suggestions
The success of the monovision technique may be further improved by having your patient follow the suggestions below. Remember to advise patients to use only one ACUVUE® Theravision™ with Ketotifen Contact Lens per eye per day.
- Have a third contact lens (distance power) to use when critical distance viewing is needed.
- Have a third contact lens (near power) to use when critical near viewing is needed.
- Having supplemental spectacles to wear over the monovision contact lenses for specific visual tasks may improve the success of monovision correction. This is particularly applicable for those patients who cannot meet state driver licensing requirements with a monovision correction.
- Make use of proper illumination when carrying out visual tasks.
Success in fitting monovision can be improved by the following suggestions:
- Reverse the distance and near eyes if a patient is having trouble adapting.
- Refine the lens powers if there is trouble with adaptation. Accurate lens power is critical for presbyopic patients.
- Emphasize the benefits of clear near vision and straight ahead and upward gaze with monovision.
The decision to fit a patient with a monovision correction is most appropriately left to the Eye Care Professional in conjunction with the patient after carefully considering the patient's needs.
PATIENT COUNSELING INFORMATION (PATIENT MANAGEMENT)
Dispensing Visit
- Evaluate the physical fit and visual acuity of the lens on each eye.
- Teach the patient how to insert and remove his or her lenses.
- Explain the daily wear regimen and schedule a follow-up examination.
Provide the patient with a copy of the ACUVUE® Theravision™ with Ketotifen patient instructions. Review these instructions with the patient so that he or she clearly understands the prescribed wearing and replacement schedule.
Review with the patient that no cleaning or disinfection is needed with daily disposable lenses. Patients should always dispose of lenses when they are removed and have spectacles or non-medicated lenses available.
Follow-Up Examinations
Follow-up care (necessary to ensure continued successful contact lens wear) should include routine periodic progress examinations, management of specific problems, if any, and a review with the patient of the wear schedule, lens replacement schedule, and proper lens care and handling procedures.
Recommended Follow-up Examination Schedule for ACUVUE® Theravision™ with Ketotifen for Daily Disposable Wear: (complications and specific problems should be managed on an individual patient basis):
- One week from the initial lens dispensing to patient.
- One month post-dispensing.
- Every three to six months thereafter.
NOTE: Preferably, at the follow-up visits, lenses should be worn for at least six hours.
Recommended Procedures for Follow-Up Visits:
- Solicit and record patient's symptoms, if any.
- Measure visual acuity monocularly and binocularly at distance and near with the contact lenses.
- Perform an over-refraction at distance and near to check for residual refractive error.
- With the biomicroscope, judge the lens fitting characteristics (as described in the "General Fitting Guidelines") and evaluate the lens surface for deposits and damage.
- Following lens removal, examine the cornea and conjunctiva with the biomicroscope and fluorescein.
- The presence of vertical corneal striae in the posterior central cornea and/or corneal neovascularization is indicative of excessive corneal edema.
- The presence of corneal staining and/or limbal-conjunctival hyperemia can be indicative of an unclean lens, a reaction to solution preservatives, excessive lens wear and/or a poorly fitting lens.
- Papillary conjunctival changes may be indicative of an unclean and/or damaged lens.
- Periodically perform keratometry and spectacle refractions. The values should be recorded and compared to the baseline measurements.
If any observations are abnormal, use professional judgment to alleviate the problem and restore the eye to optimal conditions. If the criteria for successful fit are not satisfied during any follow-up examinations, repeat the patient's trial fitting procedure and refit the patient.
WEARING SCHEDULE (DAILY WEAR)
DOSAGE AND ADMINISTRATION
- Insert one lens per eye per day. Discard lens after a single day's use.
- If prevention or relief of itching is needed beyond twelve hours, remove ACUVUE® Theravision™ with Ketotifen and consult your eye care professional.
- ACUVUE® Theravision™ with Ketotifen may be worn beyond twelve hours for vision correction. Lenses should be removed prior to sleeping.
ADDITIONAL INFORMATION
- The maximum daily wearing time should be determined by the Eye Care Professional based upon the patient's individual response to contact lenses. Regular checkups, as determined by the Eye Care Professional, are also extremely important.
- Patients tend to over wear the lenses initially. The Eye Care Professional should emphasize the importance of adhering to the initial maximum daily wearing time. Maximum wearing time should be determined by the Eye Care Professional based upon the patient's physiological eye condition, because individual response to contact lenses varies.
- ACUVUE® Theravision™ with Ketotifen is intended to be worn once on a daily disposable basis (less than 24 hours, while awake) and should be discarded upon removal. Studies have not been performed to show that ACUVUE® Theravision™ with Ketotifen is safe to wear during sleep.
- Patients should never wear lenses beyond the period recommended by the Eye Care Professional.
REPLACEMENT SCHEDULE
- These lenses are indicated for disposable wear and should be discarded upon removal.
LENS CARE DIRECTIONS
For Lenses Prescribed for Single Use Disposable Wear:
- The Eye Care Professional should review with patients that no cleaning or disinfection is needed with disposable lenses. Patients should always dispose of lenses when they are removed and have replacement non-medicated lenses or spectacles available.
Care for Sticking (Non-Moving) Lenses
- During removal, if the lens sticks to the eye, the patient should be instructed to apply a few drops of non-preserved sterile saline solution directly to the eye and wait until the lens begins to move freely on the eye before removing it. If non-movement of the lens continues after a few minutes, the patient should immediately contact the Eye Care Professional.
STORAGE CONDITIONS
Store at 15°C to 25°C (59°F to 77°F); with excursions permitted up to 30°C (86°F).
Protect from light. Store lenses in carton until use.
EMERGENCIES
The patient should be informed that if chemicals of any kind (household products, gardening solutions, laboratory chemicals, etc.) are splashed into the eyes, the patient should: FLUSH EYES IMMEDIATELY WITH TAP WATER AND IMMEDIATELY CONTACT THE EYE CARE PROFESSIONAL OR VISIT A HOSPITAL EMERGENCY ROOM WITHOUT DELAY.
REPORTING OF ADVERSE REACTIONS
All serious adverse experiences and adverse reactions observed in patients wearing these lenses or experienced with the lenses should be reported to:
Johnson & Johnson Vision Care, Inc.
7500 Centurion Parkway
Jacksonville, Florida 32256, USA
www.acuvue.com
©Johnson & Johnson Vision Care, Inc. 2021
In Canada: Johnson & Johnson Vision Care, division of Johnson & Johnson, Inc.
In USA: Johnson & Johnson Vision Care, Inc.
Printed in USA
Revision date: 2/2022
Revision number: XXXXXX
ACUVUE® Theravision™ is a trademark of Johnson & Johnson Vision Care, Inc.
ACUVUE®
Theravision™ with Ketotifen
(etafilcon A drug-eluting contact lens with ketotifen)
Contact lenses for daily wear (1-Day)
Contents: 30 etafilcon A drug-eluting contact lenses (59% H2O) in a buffered ketotifen solution. Each lens contains 19 mcg ketotifen.
PATIENT INSTRUCTION GUIDE
Rx Only
CAUTION: US Federal law restricts this device to sale by or on the order of a licensed practitioner
Dosage: See prescribing information
Light sensitive. Store blister packages in the carton until use
 USA= Johnson & Johnson Vision Care, Inc., Jacksonville, FL 32256, USA
USA= Johnson & Johnson Vision Care, Inc., Jacksonville, FL 32256, USA
IRELAND= Johnson & Johnson Vision Care Ireland UC, Limerick, Ireland
© 2021 Johnson & Johnson Vision Care Companies
www.acuvue.com
SYMBOLS KEY
The following symbols may appear on the label or packaging:
| SYMBOL | DEFINITION |
|---|---|
 | Caution, Consult Instructions for Use |
 | Manufacturer |
 | Date of Manufacture |
 | Use by Date (expiration date) |
 | Batch Code |
 | Sterilized Using Steam Heat |
 | Do Not Re-Use (Single Use) |
 | Do Not Use if Package is Damaged |
 | Store Away from Direct Sunlight |
 | Indicates a Single Sterile Barrier System |
 | Authorized Representative in the European Community |
 | Medical Device in the European Community |
 | Fee Paid for Waste Management |
 | CE-mark and Identification Number of Notified Body |
 | Contains Hazardous Substances |
 | Contains a Medicinal Substance |
 | CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed practitioner |
| CONTACT LENS | Contact Lens |
 | Lens Orientation Correct |
 | Lens Orientation Incorrect (Lens Inside Out) |
 | Package Opening Icon (Blister) |
| DIA | Diameter |
| BC | Base Curve |
| D | Diopter (Lens Power) |
INDICATIONS
ACUVUE® Theravision™ with Ketotifen are daily wear, daily disposable drug-eluting contact lenses containing an antihistamine to prevent ocular itch due to allergic conjunctivitis and correction of refractive ametropia (myopia and hyperopia) in patients who do not have red eye(s), are suitable for contact lens wear and do not have more than 1.00 D of astigmatism. Itchy eye prevention has been demonstrated to last up to 12 hours in clinical trials; however, the lens may be worn for longer than 12 hours for vision correction.
WEARING INSTRUCTIONS
DO NOT WEAR YOUR LENSES WHILE SLEEPING.
The ACUVUE® Theravision™ with Ketotifen described in this booklet are prescribed by your Eye Care Professional for daily disposable wear and are to be discarded after each removal. You should:
- Insert one lens per eye per day. Discard lens after a single day's use.
- If prevention or relief of itching is needed beyond twelve hours, consult your Eye Care Professional.
- These lenses may be worn beyond twelve hours for vision correction. Lenses should be removed prior to sleeping.
- Do not use contact lens cleaning and disinfectant solutions with ACUVUE® Theravision™ with Ketotifen.
WHEN LENSES SHOULD NOT BE WORN (CONTRAINDICATIONS)
DO NOT USE these lenses when you have any of the following conditions:
- Red or irritated eye(s). Remove contact lens(es) immediately if eye(s) become red while wearing;
- Inflammation or infection in or around the eye or eyelids
- Any eye disease, injury, or abnormality that affects the cornea, conjunctiva, or eyelids
- Any previously diagnosed condition that makes contact lens wear uncomfortable
- Severe dry eye
- Reduced corneal sensitivity
- Any medical condition that may affect the eye or be made worse by wearing contact lenses
- Allergic reactions on the surface of the eye or surrounding tissues that may be induced or made worse by wearing contact lenses
- Any active eye infection
- Known hypersensitivity to any ingredient in this product
WARNINGS
What You Should Know About Contact Lens Wear:
EYE PROBLEMS, INCLUDING CORNEAL ULCERS, CAN DEVELOP RAPIDLY AND LEAD TO LOSS OF VISION. IF YOU EXPERIENCE:
- Eye Discomfort,
- Excessive Tearing,
- Vision Changes,
- Loss of Vision,
- Eye Redness, or
- Other Eye Problems,
YOU SHOULD IMMEDIATELY REMOVE THE LENSES, AND PROMPTLY CONTACT YOUR EYE CARE PROFESSIONAL.
- These lenses should not be used to treat red eye(s). Remove lens(es) immediately if your eyes become red or irritated.
- Lenses prescribed for daily disposable wear (i.e., your Eye Care Professional instructs you to remove and discard your lenses at the end of each day), should not be worn while sleeping. Clinical studies have shown the risk of serious eye problems is increased when lenses are worn overnight.
- Contact lens wearers who are smokers have a higher incidence of corneal ulcers than nonsmokers.
- Problems with contact lenses or lens care products could result in serious injury to the eye.
- Proper use and care of your contact is essential for the safe use of these products.
- The overall risk of serious eye problems may be reduced by carefully following directions for lens care.
Specific Instructions for Use and Warnings:
Water Activity
Instruction for Use
Do not expose your contact lenses to water while you are wearing them.
Water can harbor microorganisms that can lead to severe infection, vision loss, or blindness. If your lenses have been submersed in water when participating in water sports or swimming in pools, hot tubs, lakes, or oceans, you should discard them and replace them with a new pair. Ask your Eye Care Professional for recommendations about wearing your lenses during any activity involving water.
PRECAUTIONS
For your eye health, it is important to carefully follow the handling, insertion, removal, and wearing instructions in this booklet, as well as those prescribed by your Eye Care Professional (see "Lens Handling & Insertion", "Lens Wearing" and "Caring for your Lenses" sections).
General Precautions:
Do Not use contact lens care solutions with this product
If you wear your contact lenses to correct presbyopia using monovision you may not be able to get the best corrected visual acuity for either far or near vision. Visual needs are different for different people, so your Eye Care Professional should work with you when selecting the most appropriate type of lens for you.
Always contact your Eye Care Professional before using any medicine in your eyes.
Be aware that certain medications, such as antihistamines, decongestants, diuretics, muscle relaxants, tranquilizers, oral contraceptives, and those for motion sickness may cause dryness of the eye, increased lens awareness (feeling of the lens in the eye), or blurred vision. Always inform your Eye Care Professional if you experience any problems with your lenses while taking such medications.
Do not change lens type (e.g. brand name, etc.) or parameters (e.g. diameter, base curve, lens power, etc.) without consulting your Eye Care Professional.
Consult your Eye Care Professional or Health Care Provider if you are pregnant or nursing a baby.
As with any contact lens, follow-up visits are necessary to assure the continuing health of your eyes. Ask your Eye Care Professional about the recommended follow-up schedule.
Who Should Know That You are Wearing Contact Lenses:
Inform all of your doctors (Health Care Professionals) about being a contact lens wearer.
Always inform your employer of being a contact lens wearer. Some jobs may require use of eye protection equipment or may require that you not wear contact lenses.
(POSSIBLE PROBLEMS WITH LENS WEAR AND WHAT TO DO)
Possible Problems
The most commonly observed ocular adverse reactions in clinical studies, occurring in < 2% of treated eyes, were eye irritation, eye pain, and instillation site irritation.
Other potential lens related side effects are discussed in greater detail in other sections of this guide:
Risk of Developing Corneal Ulcers (See WARNINGS)
Other Contact Lens Related Problems
Be aware that problems can occur while wearing contact lenses and may be associated with the following symptoms:
- burning, stinging, itchy, and/or dry eyes
- reduced lens comfort
- feeling of something in your eye (foreign body, scratched area)
- swelling or inflammation in or around the eyes
- eye redness
- eyelid problems
- watery eyes and/or unusual eye secretion
- poor vision
- blurred vision
- rainbows or halos around objects
- sensitivity to light (photophobia)
When any of the above symptoms occur, a serious eye condition may be present. You should immediately be seen by your Eye Care Professional, so that the problem can be identified and treated, if necessary, in order to avoid serious eye damage.
This product should not be used to treat or prevent lens-related symptoms including irritation, discomfort, or redness.
Recognizing Problems and What To Do
You should conduct a simple 3-part self-examination at least once a day. Ask yourself:
- -
- How do the lenses feel on my eyes?
- -
- How do my eyes look?
- -
- Have I noticed a change in my vision?
If you notice any problems, you should IMMEDIATELY REMOVE YOUR LENS AND CONTACT YOUR EYE CARE PROFESSIONAL.
For your eye health, it is important to carefully follow the handling, insertion, removal, and wearing instructions in this booklet, as well as those prescribed by your Eye Care Professional. If you will not or cannot always follow the recommended care procedures, you should not attempt to wear contact lenses.
When you first get your lenses, be sure that you are able to put the lenses on and remove them (or have someone else available who can remove the lenses for you) before leaving your Eye Care Professional's office.
Step 1: Getting Started
It is essential that you learn and use good hygiene in the care and handling of your new lenses.
Cleanliness is the first and most important aspect of proper contact lens care. In particular, your hands should be clean, dry, and free of any soaps, lotions, or creams before you handle your lenses.
Before you start:
Always wash your hands thoroughly with a mild soap, rinse completely, and dry with a lint-free towel before touching your lenses.
You should avoid the use of soaps containing cold cream, lotion, or cosmetics before handling your lenses. These substances may come into contact with the lenses and interfere with successful wearing. It is best to put on your lenses before putting on makeup
Step 2: Opening the Packaging
Multi-pack
Always confirm the lens parameters (e.g. diameter (DIA), base curve (BC), lens power (D), etc.) printed on the multi-pack and on the individual lens package match your prescription. DO NOT use if there is a mismatch.
Each multi-pack contains individually packaged lenses. Each lens comes in its own lens package designed specifically to keep it sterile while sealed.
These lenses are sensitive to light – store individual blister packages in the carton until use.
Lens Package
DO NOT use if the sterile blister package is opened or damaged.
To open an individual lens package, follow these simple steps:
- Shake the lens package and check to see that the lens is floating in the solution.
- Carefully peel back the foil closure to reveal the lens.
- Place a finger on the lens and slide the lens up the side of the bowl of the lens package until it is free of the container.
Occasionally, a lens may stick to the inside surface of the foil when opened, or to the plastic package itself. This will not affect the sterility of the lens. It is still perfectly safe to use. Carefully remove and inspect the lens following the handling instructions.
NEVER use tweezers or other tools to remove your lenses from the lens container.
Lens Handling Tips
- Handle your lenses with your fingertips, and be careful to avoid contact with fingernails. It is helpful to keep your fingernails short and smooth.
- Develop the habit of always working with the same lens first to avoid mix-ups.
- After you have removed the lens from the packaging, examine it to be sure that it is a single, moist, clean lens that is free of any nicks or tears. If the lens appears damaged, DO NOT use it.
Step 3: Placing the Lens on the Eye
Remember, always start with the same eye.
Once you have opened the lens package, removed, and examined the lens, follow these steps to insert the lens into your eye:
- BE SURE THE LENS IS NOT INSIDE-OUT by following one of the following procedures:
- Place the lens on the tip of your index finger and check its profile. The lens should assume a natural, curved, bowl-like shape. If the lens edges tend to point outward, the lens is inside out.
- Gently squeeze the lens between the thumb and forefinger. The edges should turn inward. If the lens is inside out, the edges will turn slightly outward.
OR - Place the lens on the tip of your index finger and, looking up at the lens, locate the numbers 1-2-3. 1-2-3 indicates correct orientation, while a reverse of 1-2-3 indicates the lens is inside out. If the lens is inside out (reverse 1-2-3), invert the lens and locate the numbers again to confirm correct lens orientation.
- With the lens on your index finger, use your other hand to hold your upper eyelid so you won't blink.
- Pull down your lower eyelid with the other fingers of your "inserting" hand.
- Look up at the ceiling and gently place the lens on the lower part of your eye.
- Slowly release your eyelid and close your eye for a moment.
- Blink several times to center the lens.
- Use the same technique when inserting the lens for your other eye.
There are other methods of lens placement. If the above method is difficult for you, ask your Eye Care Professional for an alternate method.
Step 4: Checking Your Lenses
After you have successfully inserted your lenses, you should ask yourself:
- Do I see well?
- How do the lenses feel on my eyes?
- How do my eyes look?
If after placement of the lens, your vision is blurred, check for the following:
The lens is not centered on the eye (see "Step 5: Centering the Lens," next in this booklet).
If the lens is centered, remove the lens (see "Removing Your Lenses") and check for the following:
- –
- Cosmetics or oils on the lens. Dispose of the lens and insert a new fresh lens.
- –
- The lens is on the wrong eye.
- –
- The lens is inside out (it would also not be as comfortable as normal). See "Step 3: Placing the Lens on the Eye."
If you find that your vision is still blurred after checking the above possibilities, remove the lens and consult your Eye Care Professional.
Note: If a lens is noticeably uncomfortable upon insertion or becomes less comfortable than when it was first inserted, remove the lens immediately and contact your Eye Care Professional. If your examination of your eyes and the lenses shows any other problems, IMMEDIATELY REMOVE YOUR LENSES AND CONTACT YOUR EYE CARE PROFESSIONAL.
A lens, which is on the cornea (center of your eye), will very rarely move onto the white part of the eye during wear. This, however, can occur if insertion and removal procedures are not performed properly. To center a lens, follow either of these procedures:
- Close your eyelids and gently massage the lens into place through the closed lids.
OR - Gently move the off-centered lens onto the cornea (center of your eye) while the eye is opened using finger pressure on the edge of the upper lid or lower lid.
While wearing your lenses, remember the following important precautions:
Hazardous Conditions
If you use aerosol (spray) products, such as hair-spray, while wearing lenses, keep your eyes closed until the spray has settled.
Avoid all harmful or irritating vapors and fumes while wearing lenses.
Never rinse your lenses in water from the tap. Tap water contains many impurities that can contaminate or damage your lenses and may lead to eye infection or injury.
Water Activity
- Do not expose your contact lenses to water while you are wearing them.
Lubricating/Rewetting Solutions
Lubricating/Rewetting Solutions should not be used with these lenses. If the lens sticks (stops moving), a few drops of non-preserved sterile saline may be applied to assist with removal.
Do not use saliva or anything other than the recommended solutions for lubricating or rewetting your lenses. Do not put lenses in your mouth.
Sharing Lenses
Never allow anyone else to wear your lenses. Sharing lenses greatly increases the chance of eye infections.
Adhering to the Prescribed Wearing & Replacement Schedules
Never wear your lenses beyond the amount of time recommended by your Eye Care Professional. Never wear more than one lens per day.
Always throw away worn lenses as prescribed by your Eye Care Professional.
CAUTION: Always be sure the lens is on the cornea (center of your eye) before attempting to remove it. Determine this by covering the other eye. If vision is blurred, the lens is either on the white part of the eye or it is not on the eye at all. To locate the lens, inspect the upper area of the eye by looking down into a mirror while pulling the upper lid up. Then inspect the lower area by pulling the lower lid down.
- Wash, rinse, and dry your hands thoroughly. You should follow the method that is recommended by your Eye Care Professional. Below is an example of one method: the Pinch Method.
- Pinch Method:
- Step 1. Look up, slide the lens to the lower part of the eye using the forefinger.
- Step 2. Gently pinch the lens between the thumb and forefinger.
- Step 3. Remove the lens.
- Follow the instructions in the next section, "Caring for Your Lenses".
NOTE: For your eye health, it is important that the lens moves freely on your eye. If the lens sticks (stops moving) on your eye, apply a few drops of the recommended rewetting solution. Wait until the lens begins to move freely on the eye before removing it. If non-movement of the lens continues, you should immediately consult your Eye Care Professional.
Remember, there is no cleaning or disinfection needed with these lenses. Always dispose of lenses when they are removed and have replacement non-medicated lenses or glasses available. Any unused product or waste material should be disposed of in accordance with local requirements.
Lens Storage:
- Store these lenses at room temperature
- These lenses are sensitive to light – store individual blister packages in the carton until use.
EMERGENCIES
If chemicals of any kind (household products, gardening solutions, laboratory chemicals, etc.) are splashed into your eyes: FLUSH EYES IMMEDIATELY WITH TAP WATER AND IMMEDIATELY CONTACT YOUR EYE CARE PROFESSIONAL OR VISIT A HOSPITAL EMERGENCY ROOM RIGHT AWAY.
INSTRUCTIONS FOR THE PRESBYOPIC PATIENT
The decision to be fit with monovision correction is most appropriately left to your Eye Care Professional, in conjunction with you, after carefully considering and discussing your needs.
Johnson & Johnson Vision Care, Inc.
7500 Centurion Parkway
Jacksonville, FL 32256
USA
Tel: 1-800-843-2020
www.acuvue.com
© Johnson & Johnson Vision Care, Inc. 2021
In Canada: Johnson & Johnson Vision Care division of Johnson & Johnson Inc.
In USA: Johnson & Johnson Vision Care, Inc
Revision Date: 2/2022
Revision number: XXXXX