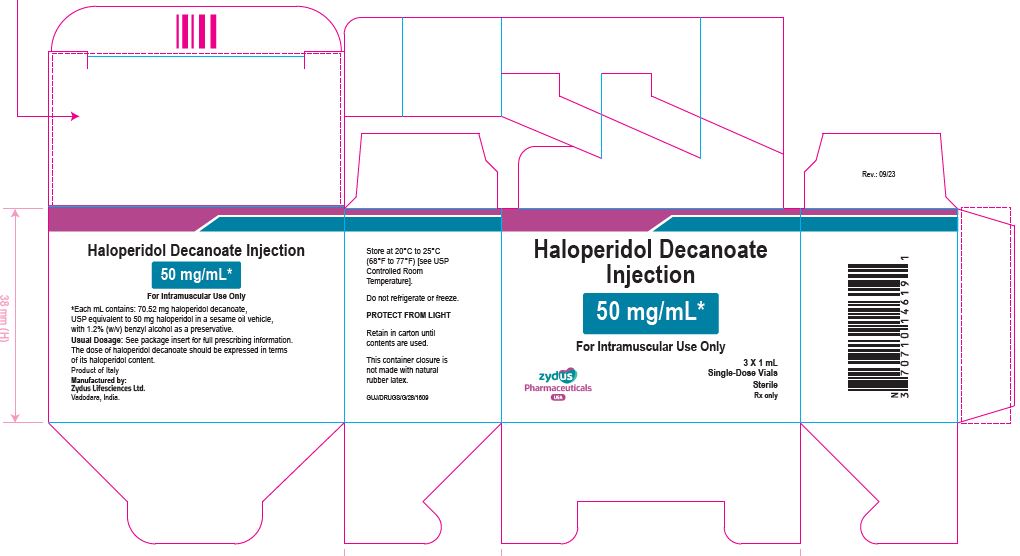
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
50 mg/mL Carton (3 vials per carton)
NDC 70771-1851-9
Haloperidol Decanoate
Injection
50 mg/mL*
For Intramuscular Use Only
3 X 1 mL
Single-Dose Vials
Sterile
Rx only
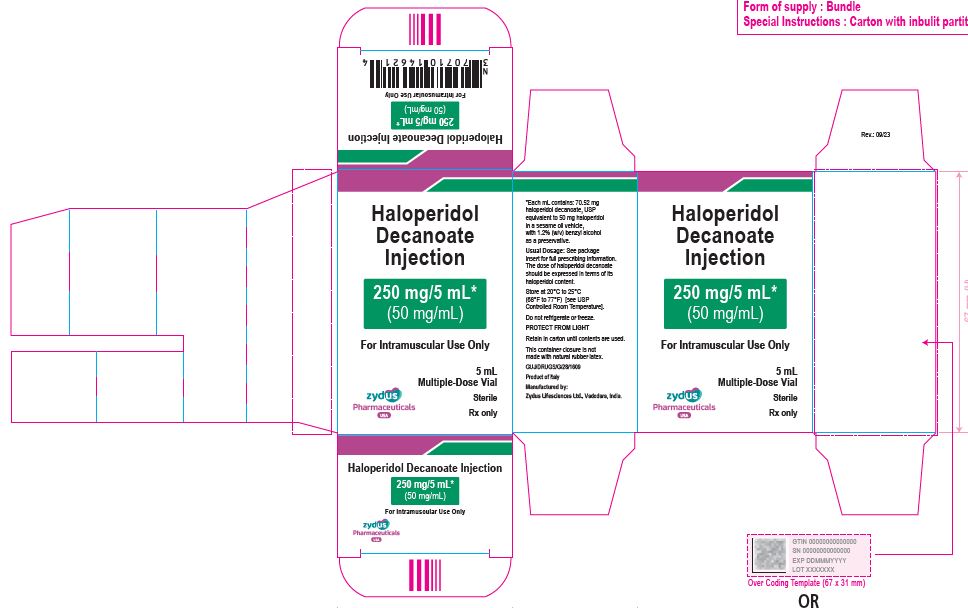
250 mg/5 mL (50 mg/mL) Carton (1 vial per carton)
NDC 70771-1852-1
Haloperidol Decanoate Injection
250 mg/5 mL*
(50 mg/mL)
For Intramuscular Use Only
5 mL
Multiple-Dose Vial
Sterile
Rx only
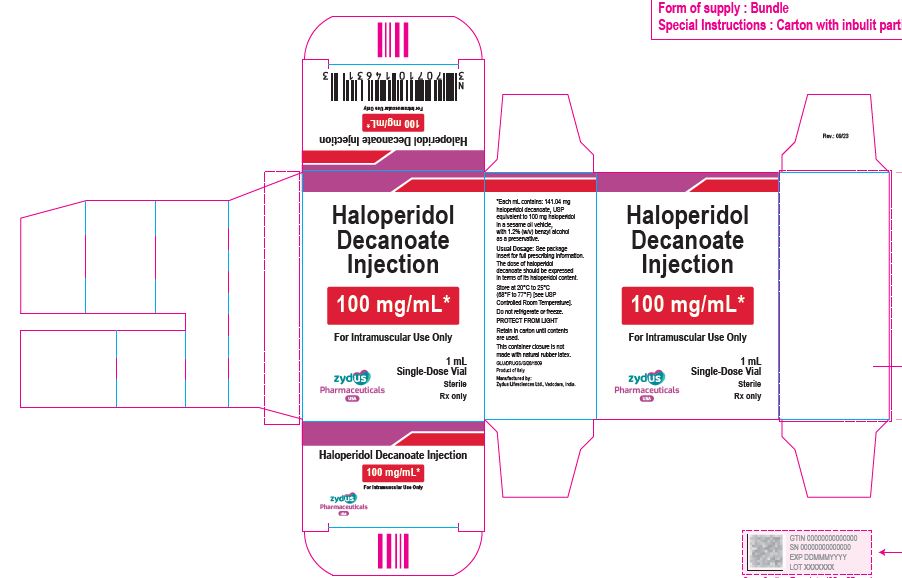
100 mg/mL Carton (1 vial per carton)
NDC 70771-1853-1
Haloperidol Decanoate Injection
100 mg/mL*
For Intramuscular Use Only
1 mL
Single-Dose Vial
Sterile
Rx only
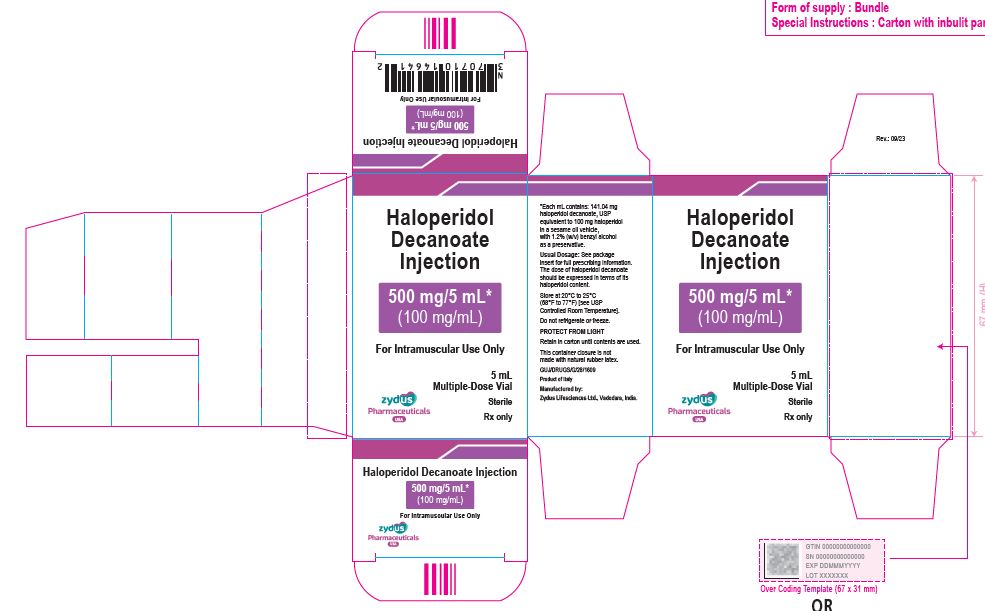
500 mg/5 mL (100 mg/mL) Carton (1 vial per carton)
NDC 70771-1854-1
Haloperidol Decanoate Injection
500 mg/5 mL*
(100 mg/mL)
For Intramuscular Use Only
5 mL Multiple-Dose Vial
Sterile
Rx only