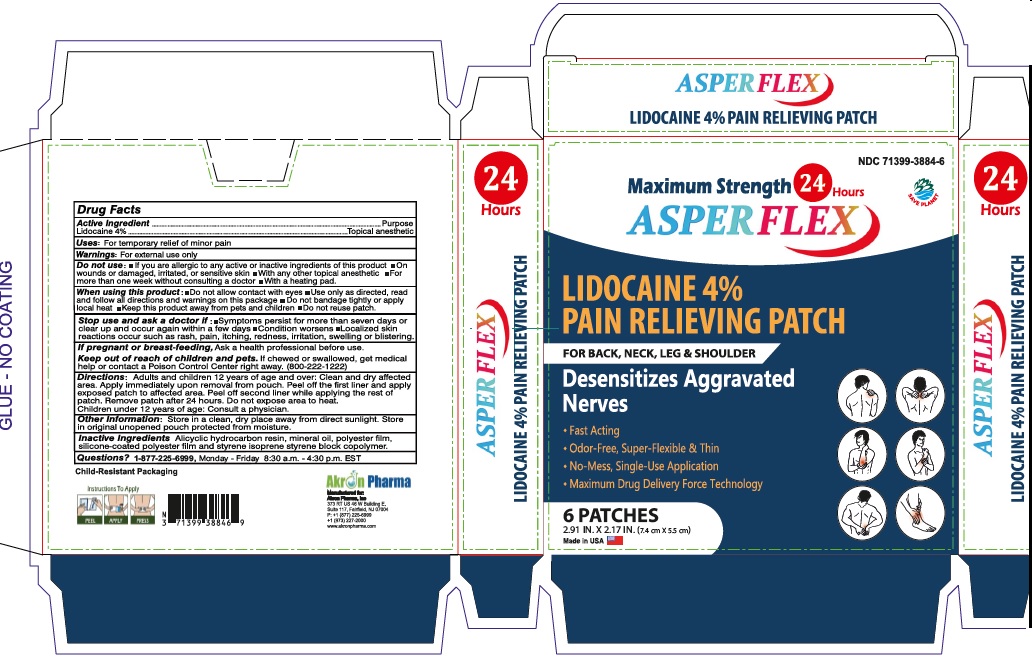
Active Ingredient
Lidocaine 4% . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Topical Anesthetic
Dosage and Administration
Directions Adults and children over 12 years:
- clean and dry affected area
- carefully remove backing from patch starting at corner
- apply sticky side of patch to affected area
- use one patch for up to 24 hours. Discard patch after single use. Children under 12 years of age: consult a physician.
When using this product
- use only as directed
- read and follow all directions and warnings on this carton
- do not allow contact with the eyes
- do not use at the same time as other topical analgesics
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
- do not microwave
- dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
Stop use and consult a doctor
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
- you experience signs of skin injury, such as pain, swelling or blistering where the product was applied.
If pregnant or breastfeeding, ask a health professional before use.
Do not Use
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
- you experience signs of skin injury, such as pain, swelling or blistering where the product was applied.
Keep out of reach of children and pets.
If swallowed, get medical help or contact a Poison Control Center right away 800-222-1222