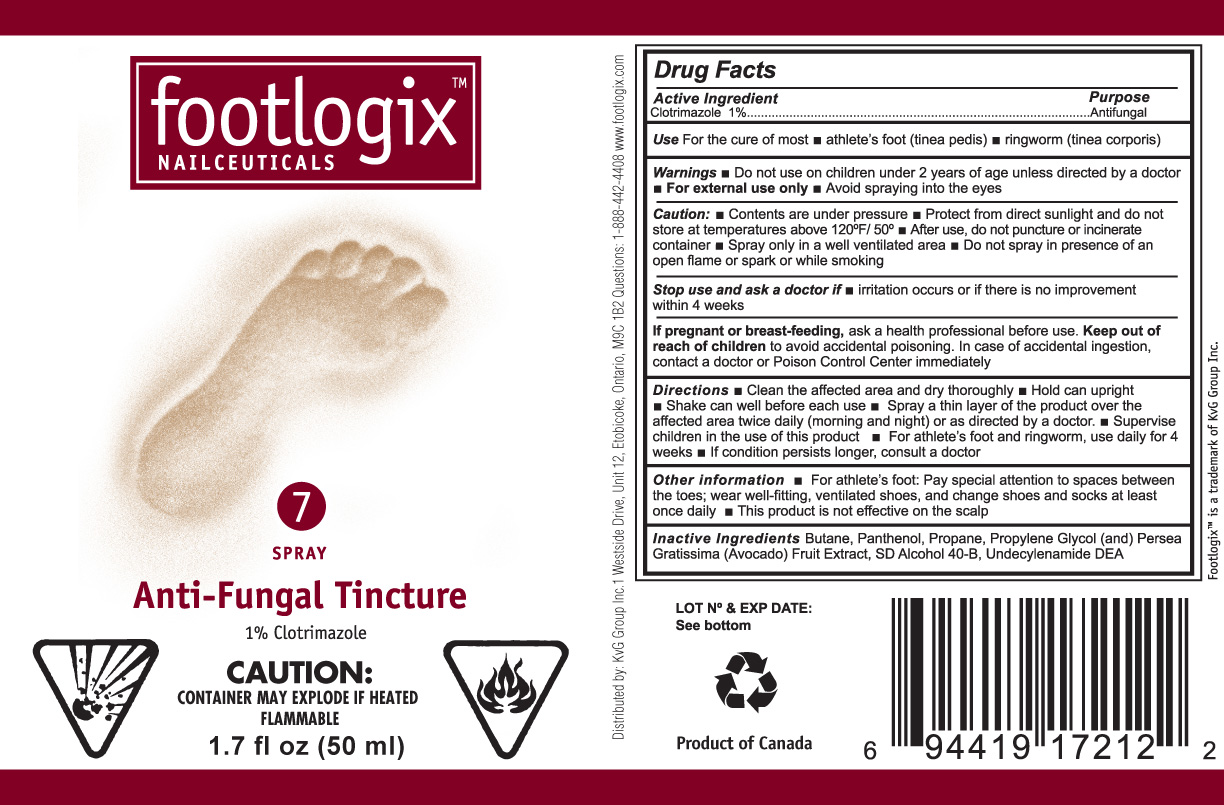
Warnings
- For external use only
- Do not use on children under 2 years of age unless directed by a doctor
- For external use only
- Avoid spraying into the eyes
Caution:
- Contents are under pressure
- Protect from direct sunlight and do not store at temperatures above 120F/50C
- After use, do not puncture or incinerate container
- Spray only in a well ventilated area
- Do not spray in presence of open flame or spark or while smoking
Keep out of reach of children to avoid accidental poisoning. In case of accidental ingestion, contact a doctor or Poison Control Center immediately.
Directions
- Clean the affected area and dry thoroughly
- Hold can upright
- Shake can well before each use
- Spray a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor.
- Supervise children in the use of this product
-
For athlete’s foot and ringworm, use
daily for 4 weeks
- If condition persists longer, consult a doctor
Other information
- For athlete’s foot Pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily.
- This product is not effective on the scalp