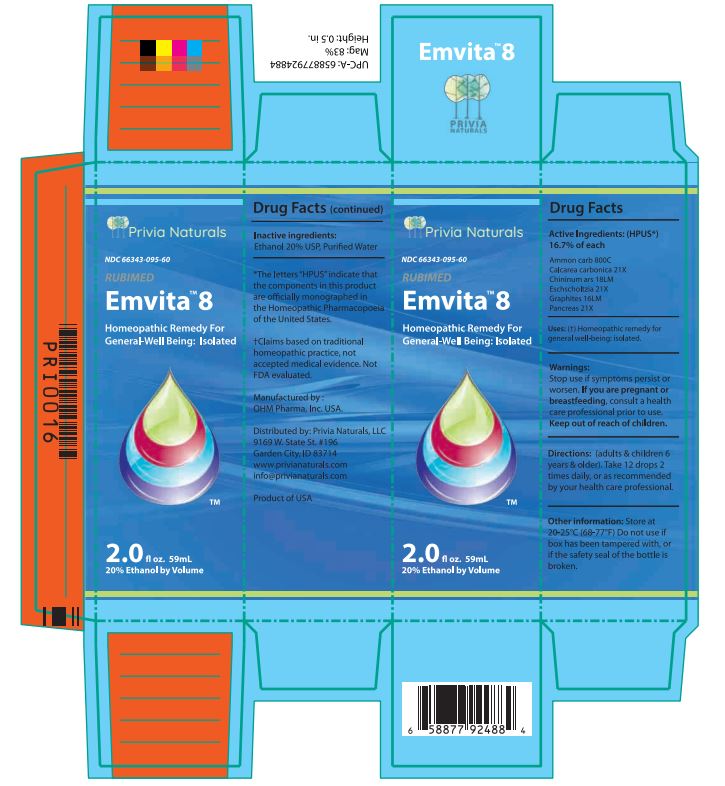
Drug Facts Active Ingredients: (HPUS*) 16.7% of each
Ammon carb 800C Calcarea carbonica 21X
Chininum ars 18LM Eschscholtzia 21X
Graphites 16LM Pancreas 21X
*The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. †Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Warnings:
Stop use if symptoms persist or worsen.
If you are pregnant or breastfeeding, consult a healthcare professional prior to use.
Directions: (adults & children 6 years & older)
Take 12 drops 2 times daily, or as recommended by your health care professional.
Other information: Store at 20 - 25°C
(68 - 77°F.) Do not use if box has been
tampered with, or if the safety seal of the
bottle is broken.