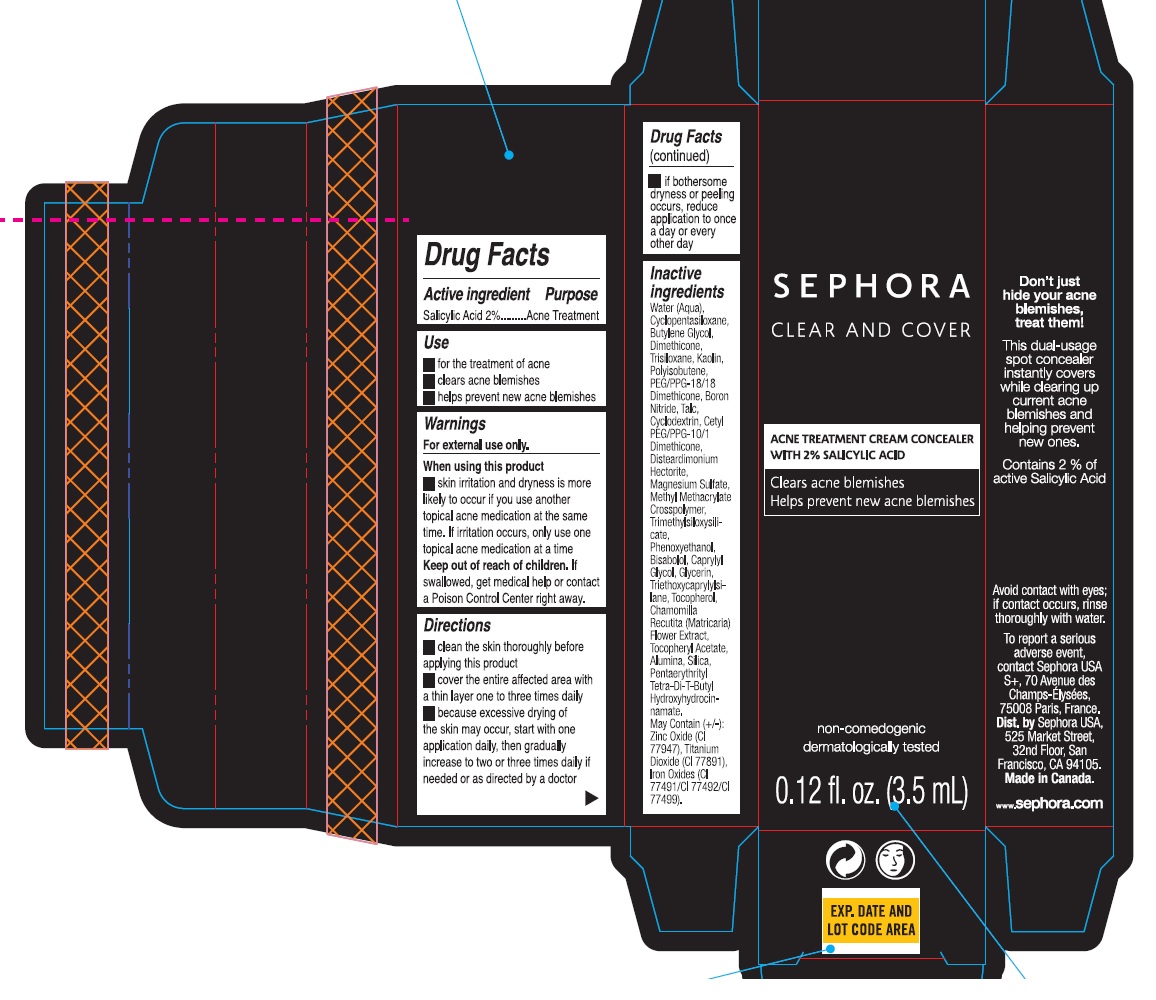
Warnings
For external use only.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- becaused excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three tiems daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
Water (Aqua), Cyclopentasiloxane, Butylene Glycol, Dimethicone, Trisiloxane, Kaolin, Polyisobutene, PEG/PPG-18/18 Dimethicone, Boron Nitride, Talc, Cyclodextrin, Cetyl PEG/PPG-10/1 Dimethicone, Disteardimonium Hectorite, Magnesium Sulfate, Methyl Methacrylate Crosspolymer, Trimethylsiloxysilicate, Phenoxyethanol, Bisabolol, Caprylyl Glycol, Glycerin, Triethoxycaprylylsilane, Tocopherol, Chamomilla Recutita (Matricaria) Flower Extract, Tocopheryl Acetate, Alumina, Silica, Pentaerythrityl Tetra-Di-T-Butyl Hydroxyhydrocinnamate.
May Contain (+/-): Zinc Oxide (CI 77947), Titanium Dioxide (CI 77891), Iron Oxides (CI 77491/CI 77492/CI 77499).