DOCUSATE SODIUM- docusate sodium capsule, liquid filled
J.P. BUSINESS ENTERPRISE
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
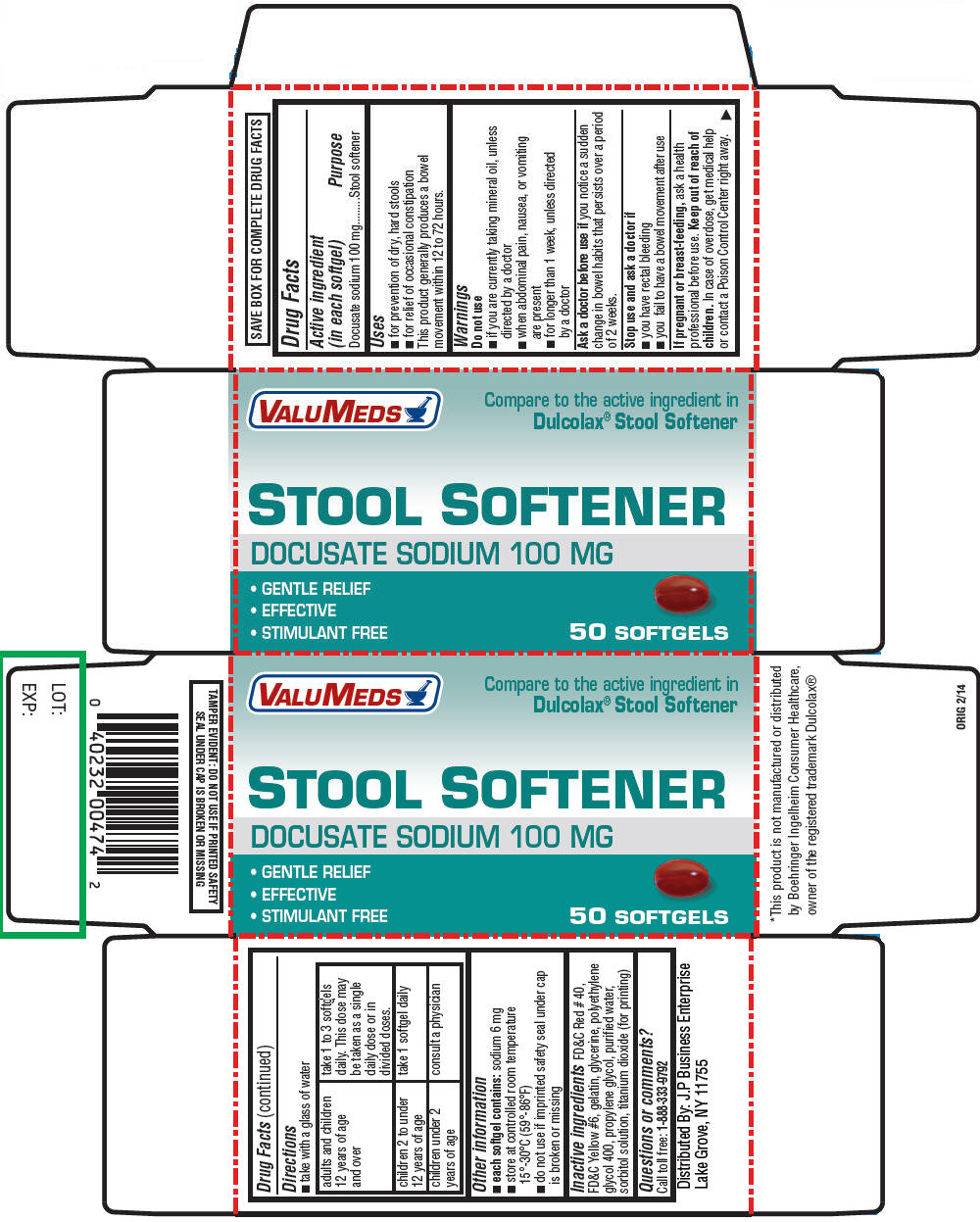
Active ingredient (in each softgel)
Docusate sodium 100 mg
Uses
- for prevention of dry, hard stools
- for relief of occasional constipation
This product generally produces a bowel movement within 12 to 72 hours.
Warnings
Do not use
- if you are currently taking mineral oil, unless directed by a doctor
- when abdominal pain, nausea, or vomiting are present
- for longer than 1 week, unless directed by a doctor
Ask a doctor before use if you notice a sudden change in bowel habits that persists over a period of 2 weeks.
Stop use and ask a doctor if
- you have rectal bleeding
- you fail to have a bowel movement after use
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- take with a glass of water
| adults and children 12 years of age and over | take 1 to 3 softgels daily. This dose may be taken as a single daily dose or in divided doses. |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years of age | consult a physician |
Other information
-
each softgel contains: sodium 6 mg
- store at controlled room temperature 15°-30°C (59°-86°F)
- do not use if imprinted safety seal under cap is broken or missing
Inactive ingredients
FD&C Red # 40, FD&C Yellow #6, gelatin, glycerine, polyethylene glycol 400, propylene glycol, purified water, sorbitol solution, titanium dioxide (for printing)
Questions or comments?
Call toll free:
1-888-333-9792
Distributed By: J.P Business Enterprise
Lake Grove, NY 11755
PRINCIPAL DISPLAY PANEL - 50 Softgel Bottle Carton
VALUMEDS
Compare to the active ingredient in
Dulcolax
® Stool Softener
STOOL SOFTENER
DOCUSATE SODIUM 100 MG
- GENTLE RELIEF
- EFFECTIVE
- STIMULANT FREE
50 SOFTGELS
J.P. BUSINESS ENTERPRISE