MENTHOL AND ZINC OXIDE- menthol and zinc oxide ointment
Dynarex Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Menthol 0.44%
Zinc Oxide 20.625%
Purpose
External Analgesic
Skin Protectant
Uses
• A moisture barrier that prevents and helps heal skin irritations from:
• Urine • Diarrhea • Perspiration • Fistula drainage • Feeding tube site leakage • Wound drainage (peri-wound skin) • Minor burns
• Cuts • Scrapes • Itching
Warnings
For external use only
When using this product
• Avoid contact with eyes
Do not use section
• On deep or puncture wounds
Stop use and ask a doctor if
• Condition worsens • Symptoms last more than 7 days or clear up and occur again within a few days
Keep Out Of Reach Of Children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
• Cleanse skin with mild skin cleanser.
• Pat dry or allow to air dry.
• Apply a thin layer of Calasoothe to reddened or irritated skin 2-4 times daily, or after each incontinent episode or diaper change to promote comfort and long lasting protection.
Other Information
• Store at room temperature 15º-30º C (59º-86º F)
• Tamper evident. Do not use if packet is torn or cut. Do not use if seal is damaged.
Inactive Ingredients
Calamine, Glycerine, Lanolin, Mineral Oil, Phenol, Sodium Bicarbonate, Thymol, White Soft Paraffin
Questions?
1-888-DYNAREX Monday - Friday, 9AM - 5PM EST
Label
 Calasoothe 4 oz. tube
Calasoothe 4 oz. tube
Label
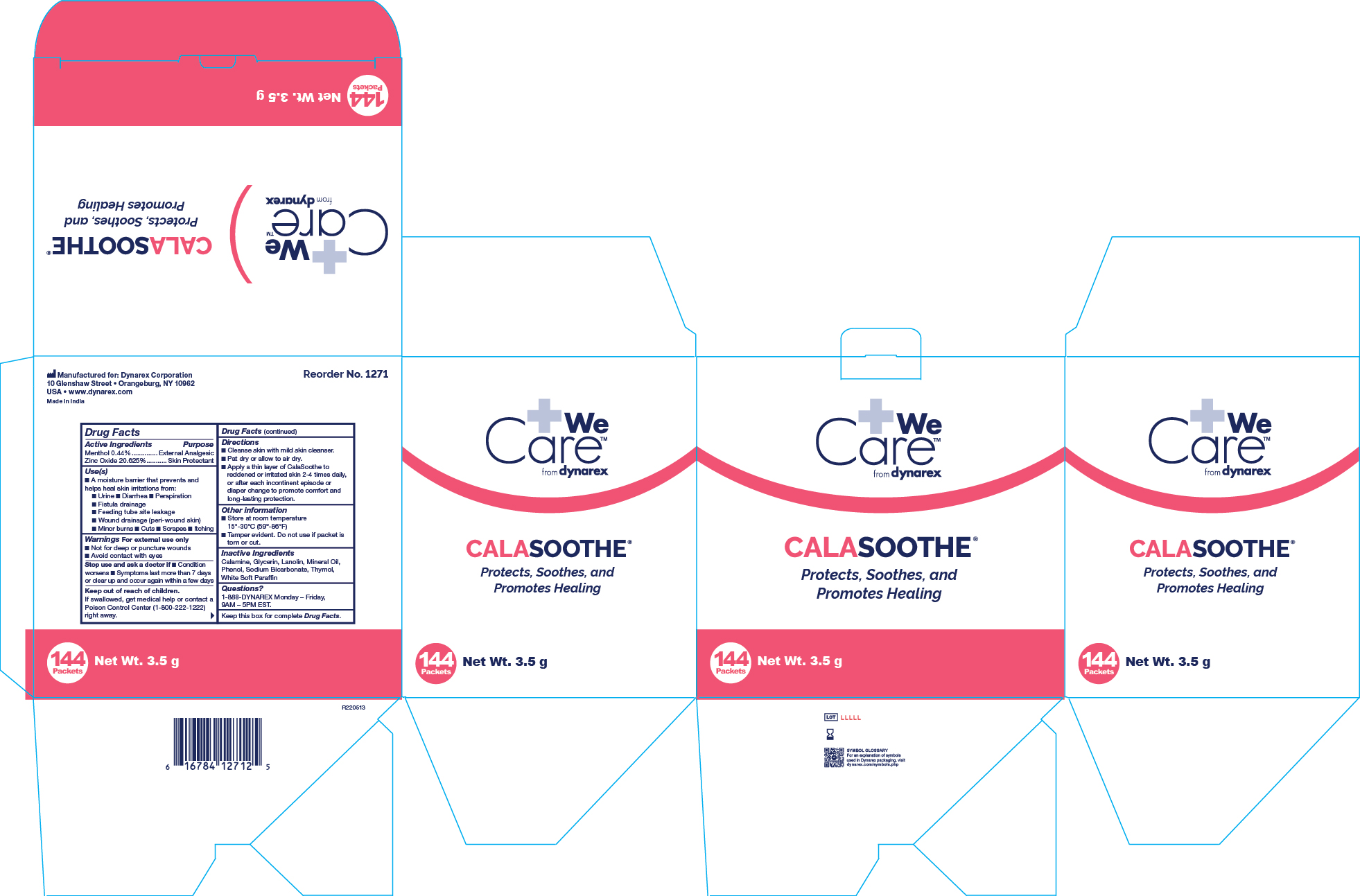 1271 Calasoothe
1271 Calasoothe
Label
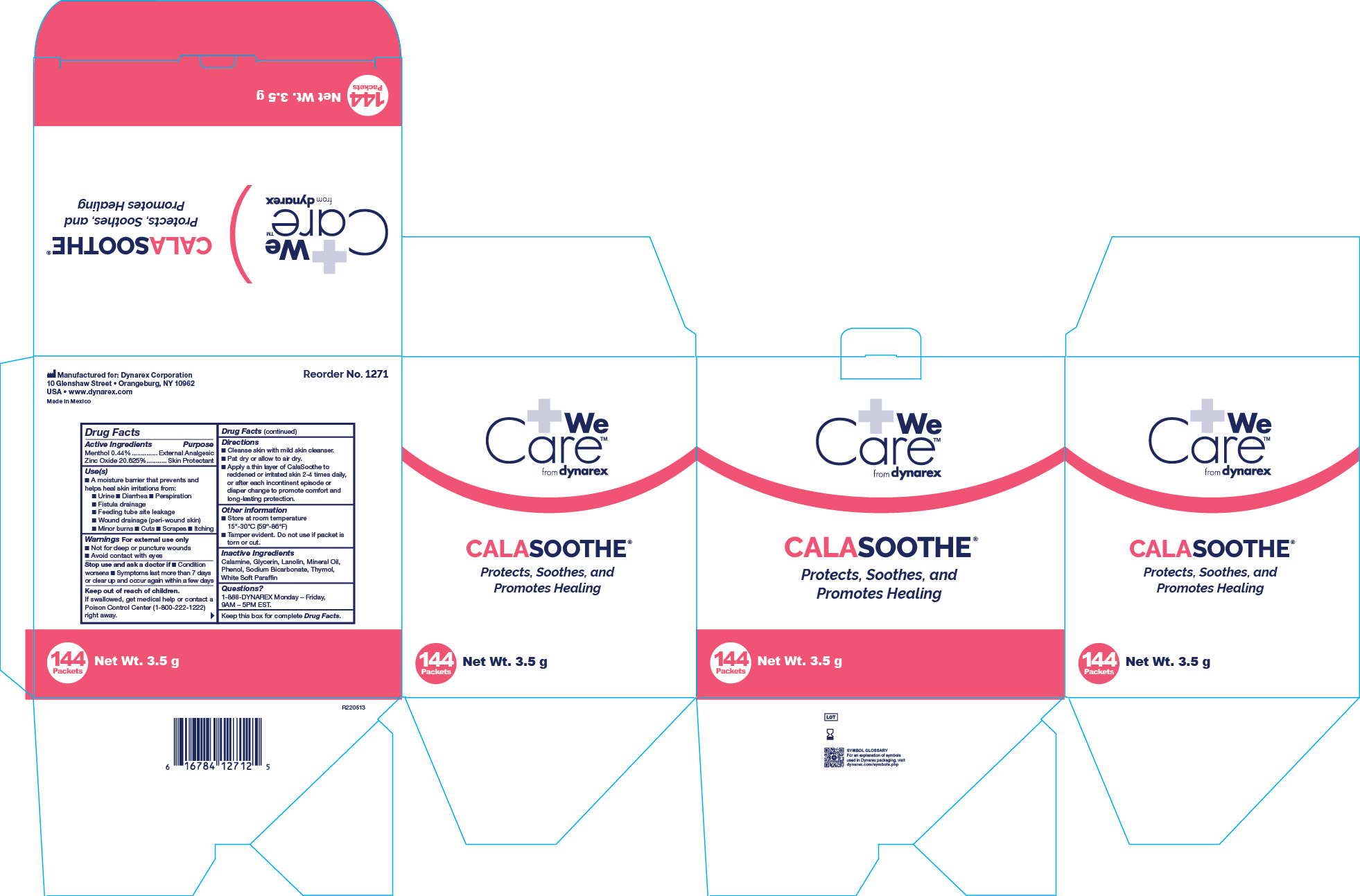 1271 Calasoothe
1271 Calasoothe
 Calasoothe 4 oz. tube
Calasoothe 4 oz. tube
 1271 Calasoothe
1271 Calasoothe
 1271 Calasoothe
1271 Calasoothe