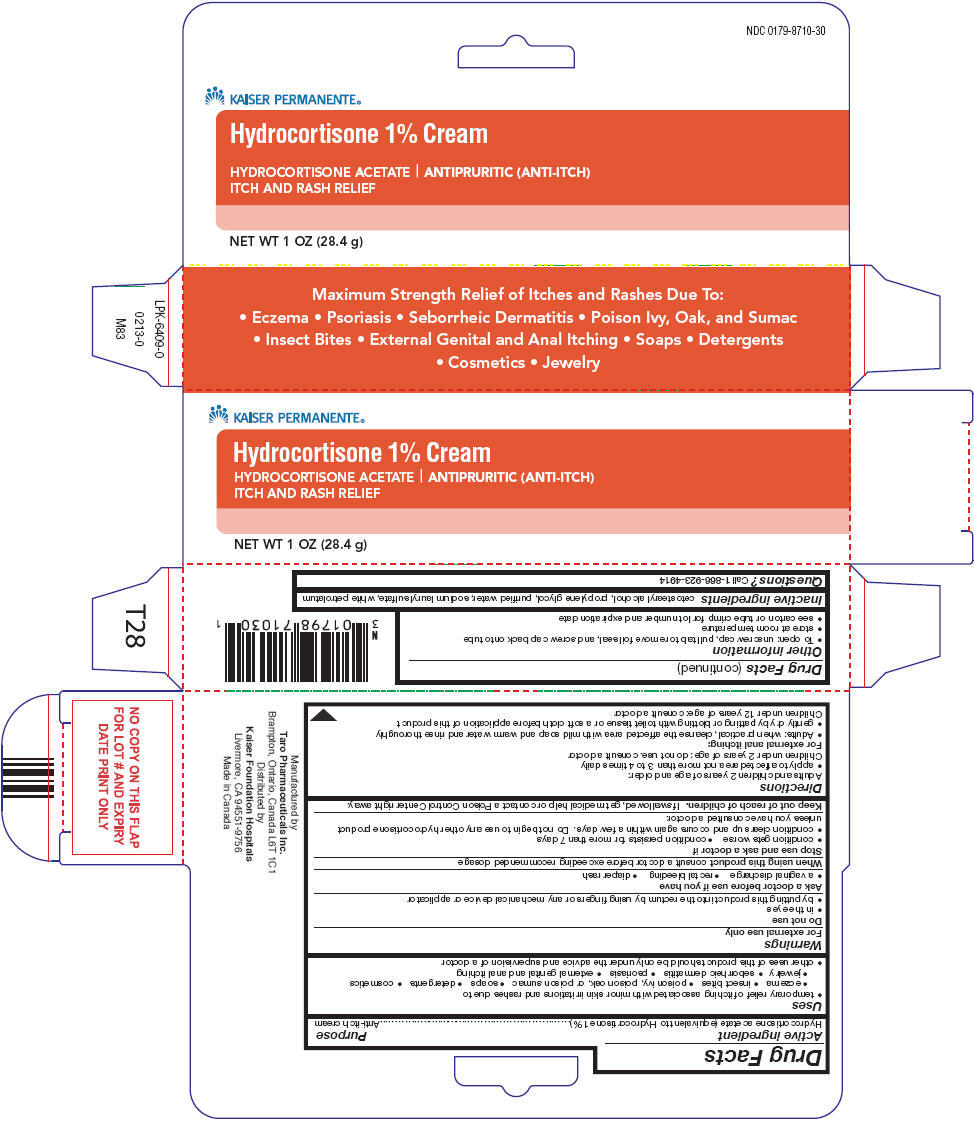
KAISER PERMANENTE HYDROCORTISONE- hydrocortisone acetate cream
Kaiser Foundation Hospitals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
KAISER PERMANENTE
®
Hydrocortisone
Uses
- temporary relief of itching associated with minor skin irritations and rashes due to
- eczema
- insect bites
- poison ivy, poison oak, or poison sumac
- soaps
- detergents
- cosmetics
- jewelry
- seborrheic dermatitis
- psoriasis
- external genital and anal itching
- other uses of this product should be only under the advice and supervision of a doctor
Warnings
For external use only
Do not use
- in the eyes
- by putting this product into the rectum by using fingers or any mechanical device or applicator
Directions
Adults and children 2 years of age and older:
- apply to affected area not more than 3 to 4 times daily
Children under 2 years of age: do not use. consult a doctor
Other information
- To open: unscrew cap, pull tab to remove foil seal, and screw cap back onto tube
- store at room temperature
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
cetostearyl alcohol, propylene glycol, purified water, sodium lauryl sulfate, white petrolatum
| KAISER PERMANENTE
HYDROCORTISONE
hydrocortisone acetate cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kaiser Foundation Hospitals (053052619) |
| Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | manufacture(0179-8710) | |
Revised: 11/2018
Document Id: 7b0ac476-1138-3470-e053-2991aa0a9a59
Set id: d0cc2907-5276-447f-9355-35e81a7e4e12
Version: 2
Effective Time: 20181119
Kaiser Foundation Hospitals