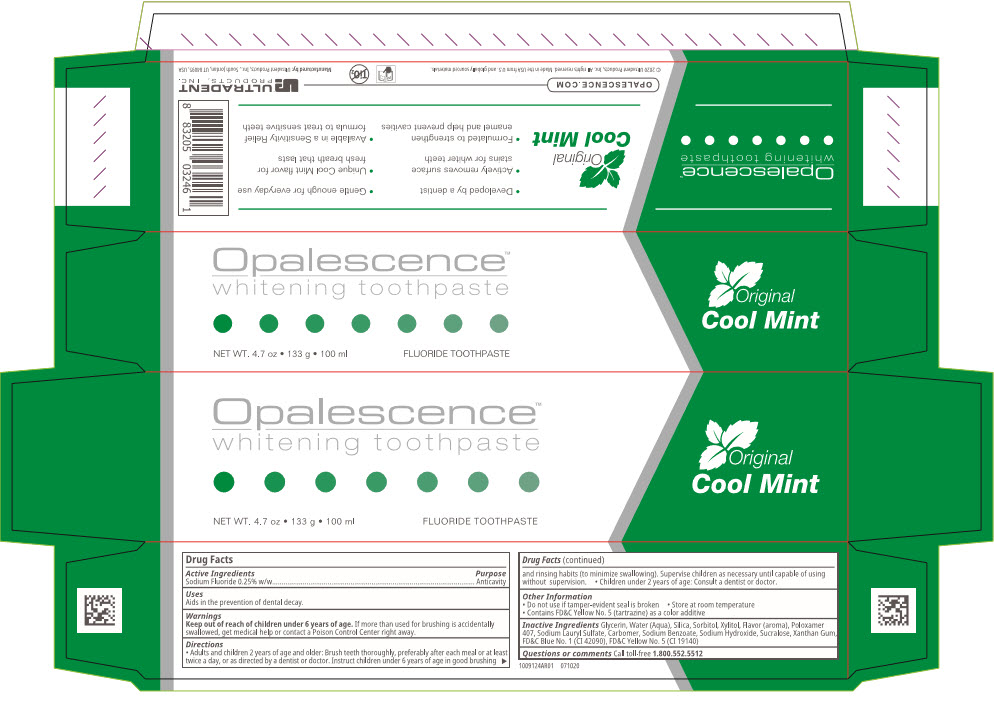
Active Ingredients
Sodium Fluoride 0.25% w/w
Uses
Aids in the prevention of dental decay.
Warnings
Keep out of reach of children under 6 years of age.If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor. Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing). Supervise children as necessary until capable of using without supervision.
- Children under 2 years of age: Consult a dentist or doctor.
Other Information
- Do not use if tamper-evident seal is broken
- Store at room temperature
- Contains FD&C Yellow No. 5 (tartrazine) as a color additive
Inactive Ingredients
Glycerin, Water (Aqua), Silica, Sorbitol, Xylitol, Flavor (aroma), Poloxamer 407, Sodium Lauryl Sulfate, Carbomer, Sodium Benzoate, Sodium Hydroxide, Sucralose, Xanthan Gum, FD&C Blue No. 1 (CI 42090), FD&C Yellow No. 5 (CI 19140)
Questions or comments
Call toll-free
1.800.552.5512
Manufactured by:Ultradent Products, Inc., South Jordan, UT 84095, USA
PRINCIPAL DISPLAY PANEL - 100 ml Tube Box
Opalescence™
whitening toothpaste
NET WT. 4.7 oz • 133 g • 100 ml
FLUORIDE TOOTHPASTE
Original
Cool Mint