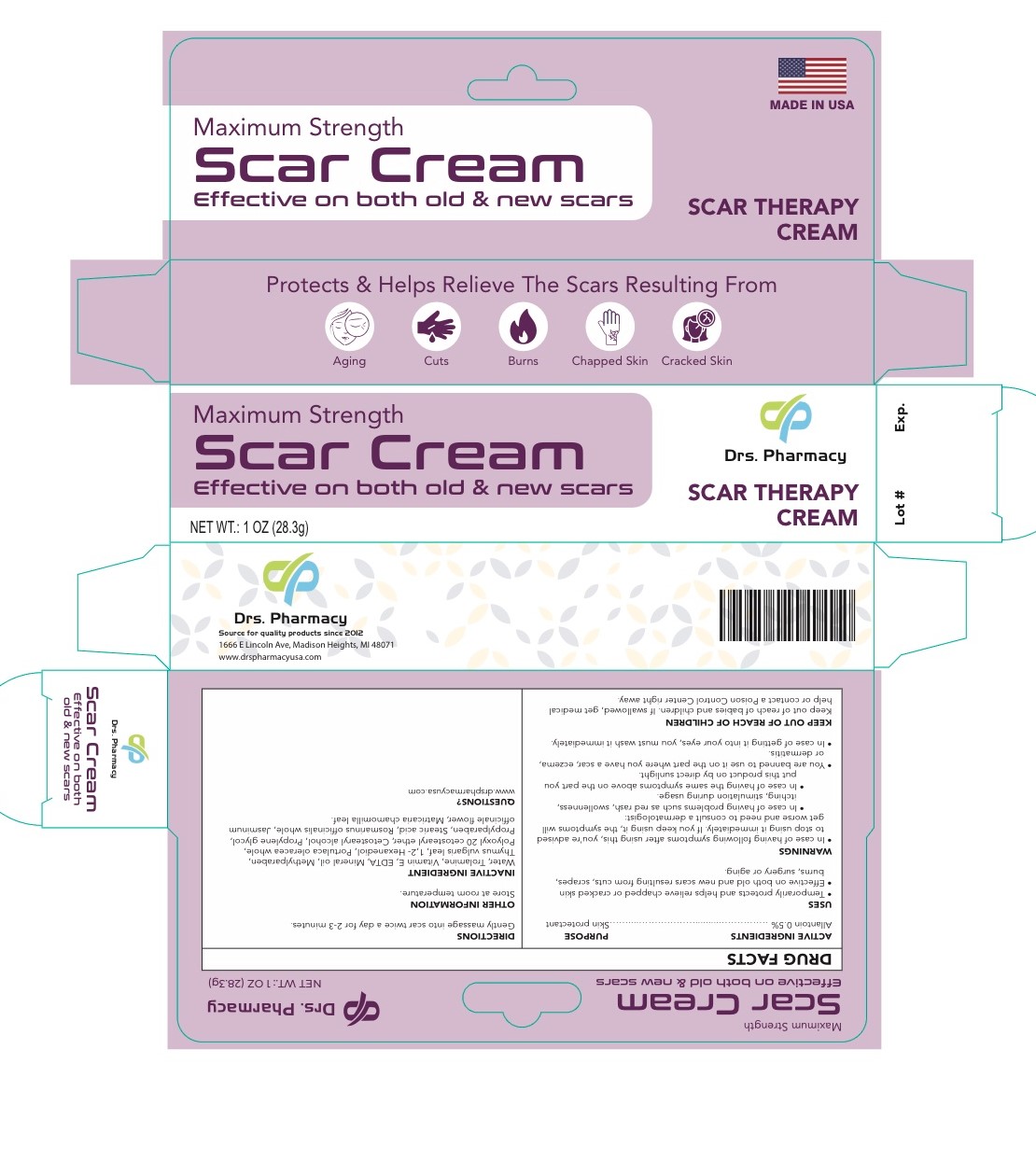
Inactive Ingredients
Cetostearyl alcohol- mineral oil- ceteareth 20- propylene glycol- methyl paraben- propylparaben- vitamin E- Stearic acid - trolamine RosmarinusOfficinalis (Rosemary)Extract/ Portulaca Oleracea Extract/Thymus Vulgaris Thyme) Leaf Extract/Jasminum Officinale (jasmine) Flower/ Leaf Extract?Chamomila) Recutita (Matricaria) Flower Water, Aureobasidium Pullulans Ferment Extract/ 1.2-Hexanedio
Warnings
- In case of having the following symptoms after using this cream, you are advised to stop using it immediately. If you keep using it , the symptoms will get worse and need to consult a drematologist .1- in case of having problems such as red rash, swollllenness, itching, stimulation during usage. 2- in case of having the same symptoms above on the part you put this product on by direct sunlight
- you are banned to use it on the part where you have a eczema, lor dermatitis.
- in case of getting it into your eyes, you have to wash it immediately.