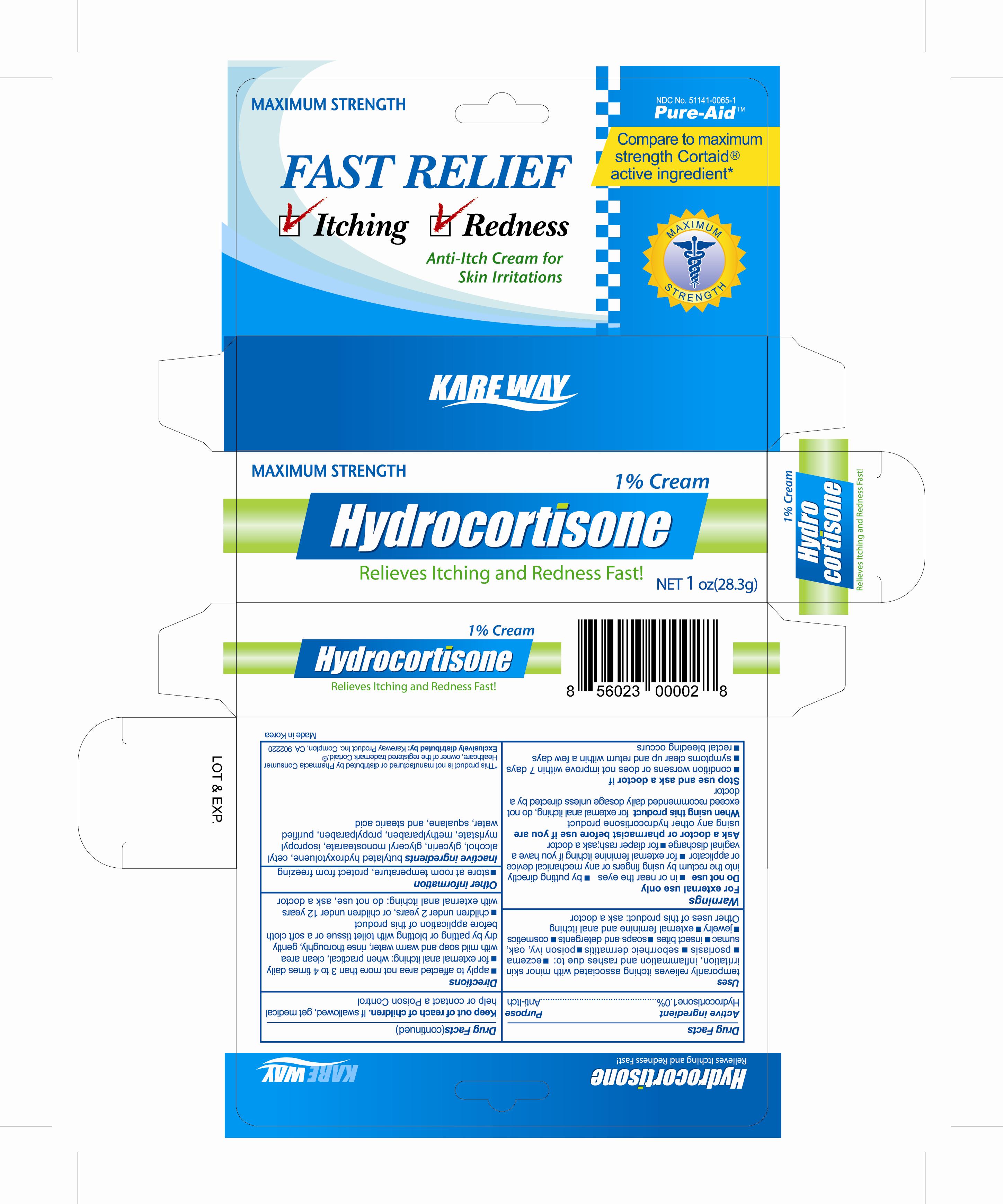
HYDROCORTISONE HC- hydrocortisone cream
Neopharm Co,. Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Hydrocortisone 1%
Keep out of reach of children
if swallowed, get medical help or contact a Poison Control
Uses
temporarily relieves itching
associated with minor skin irritation, inflammation and rashes due to :
- psoriasis
- seborrheic dermatitis
- poison ivy,oak,sumac
- insect bites
Other uses of this product: ask a doctor
Warnings
For external use only
Do not use
* in or near the eyes
* by putting directly into the rectum by using figers or any mechanical device
or applicator
* for diaper rash; ask a doctor
Directions
- apply to affected area not more than 3 to 4 time daily
- for external anal itching: when practical, clean area with mild soap and warm
Inactive ingredients
Cetyl alcohol, Buthylated hydroxytoluene, Glycerin, Glyceryl monostearate, Isopropyl myristate, Myreistoyl/palmitoyl/oxostearamide/arachamide MEA, Methylparaben, PEG-15 glyceryl stearate, Squalane, Stearic acid, Propylparaben, Purified water
Hydrocortosone
Neopharm Co,. Ltd