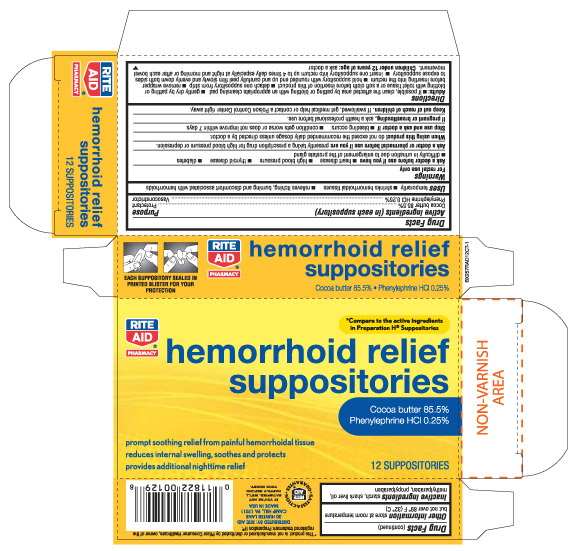
Active ingredients (in each suppository) Purpose
Cocoa Butter 85.5%........................................................................ Protectant
Phenylephrine HCL 0.25%............................................................... Vasoconstrictor
Cocoa Butter 85.5%........................................................................ Protectant
Phenylephrine HCL 0.25%............................................................... Vasoconstrictor
Active ingredients (in each suppository) Purpose
Cocoa Butter 85.5%........................................................................ Protectant
Phenylephrine HCL 0.25%............................................................... Vasoconstrictor
________________________________________________________________________________________
Uses temporarily - Shrinks hemorrhoidal tissues - relieves itching, burning and discomfort associated with hemorrhoids
Keep out of reach of children. If swallowed, get
medical help or contact a Poison Control Center right away.
Uses - shrinks hemorrhoidal tissues - relieves itching, burning and discomfort associated with hemorrhoids
Warnings
For rectal use only
____________________________________________________
Ask a doctor before use if you have - heart disease - high blood pressure
- thyroid disease - diabetes - difficulty in urination due to enlargement of the prostate gland
_____________________________________________________
Ask a doctor or pharmacist before use if you are - presently taking a prescription
drug for high blood pressure or depression
______________________________________________________
Stop use and ask a doctor if
- bleeding occurs - condition gets worse or does not improve within 7 days
______________________________________________________
If pregnant or breastfeeding, ask a health professional before use
_______________________________________________________________
Keep out of reach of children. If swallowed, get
medical help or contact a Poison Control Center
right away
______________________________________________________
For rectal use only
____________________________________________________
Ask a doctor before use if you have - heart disease - high blood pressure
- thyroid disease - diabetes - difficulty in urination due to enlargement of the prostate gland
_____________________________________________________
Ask a doctor or pharmacist before use if you are - presently taking a prescription
drug for high blood pressure or depression
______________________________________________________
Stop use and ask a doctor if
- bleeding occurs - condition gets worse or does not improve within 7 days
______________________________________________________
If pregnant or breastfeeding, ask a health professional before use
_______________________________________________________________
Keep out of reach of children. If swallowed, get
medical help or contact a Poison Control Center
right away
______________________________________________________
Directions
- Adults - if possible, clean the affected area by patting or blotting with an appropriate cleansing pad
- gently dry by patting or blotting with toilet tissue or a soft cloth before insertion of this product
- detach one suppository from strip - remove wrapper before inserting into the rectum - hold suppository
with rounded end up and carefully peel film slowly and evenly down both sides to expose the suppository
- insert one suppository into rectum up to 4 times daily especially at night and morning or after each bowel
movement
Children under 12 years of age: ask a doctor
________________________________________________________________________________________
Other information - store at room temperature but not over 89 degrees F (32 degrees C)