Active Ingredients (in each chewable tablet)
Meclizine 25 mg
Indications and Usage
prevents and treats nausea, vomiting, dizziness associated with motion sickness:
Warnings
-
Do not use for children under 12 years of age unless directed by a doctor.
Do not take unless directed by a doctor if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Do not take if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product
- do not exceed recommended dosage
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding,
- ask health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. 1-800-222-1222
Directions
Dosage should be taken 1 hour befor travel starts.
| Adults and children 12 years and over: | Take 1 or 2 tablets once daily, or as directed by doctor. |
Inactive ingredients
Croscarmellose sodium, dextrose, FD& C Red #40, flavor, magnesium stearate, microcrystalline cellulose, silicon dioxide, sodium saccharine, stearic acid
Questions?
Adverse drug event call (866) 562-2756 (M-F, 8AM-4PM EST).
How Supplied
Meclizine 25mg are supplied as chewable pink round scored tablets with PH 051 embossed on them.
Supplied in bottles of 6, 12 , 20, 30 and 100 chewable tablets.
PRINCIPAL DISPLAY PANEL - 25 mg Bottle Label
Antiemetic
Each chewable tablet contains:
Meclizine HCl
25 mg
Store at 68°-77°F (20°-25°C)
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.
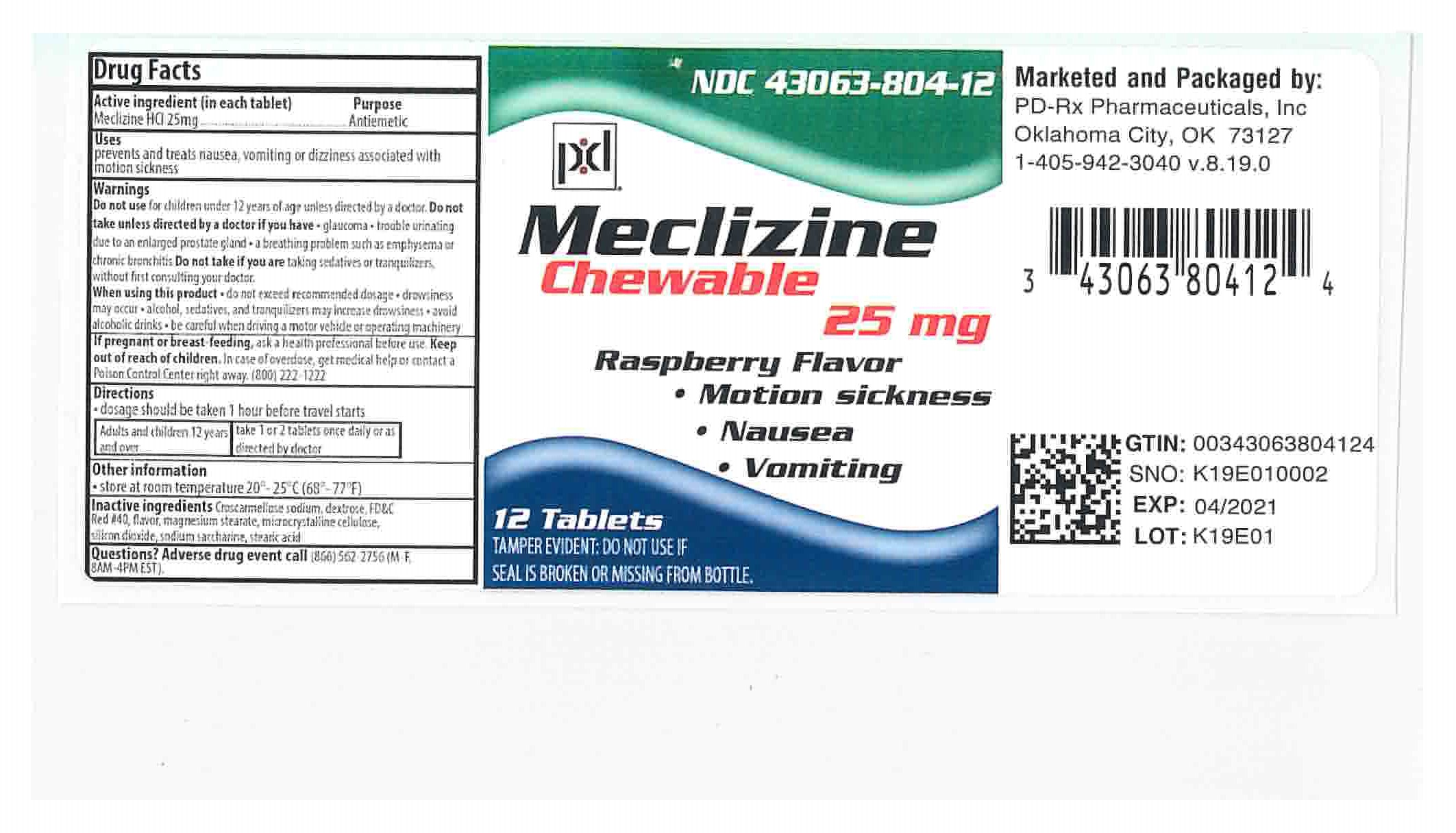
PD-Rx Pharmaceuticals, Inc.
