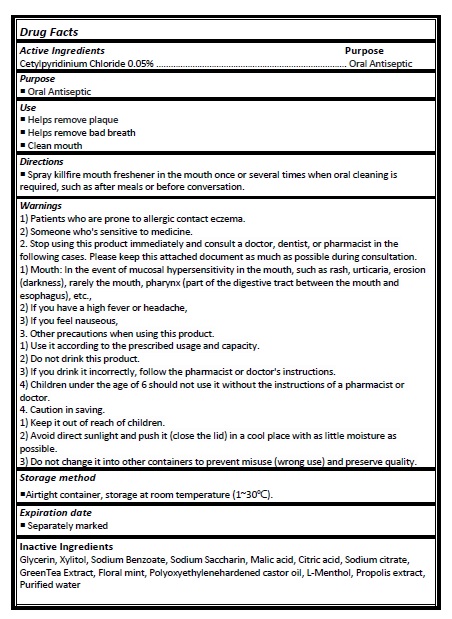
Active ingredient
Cetylpyridinium Chloride 0.05%
Directions
- Spray killfire mouth freshener in the mouth once or several times when oral cleaning is required, such as after meals or before conversation.
Warnings
1) Patients who are prone to allergic contact eczema.
2) Someone who's sensitive to medicine.
2. Stop using this product immediately and consult a doctor, dentist, or pharmacist in the following cases. Please keep this attached document as much as possible during consultation. 1) Mouth: In the event of mucosal hypersensitivity in the mouth, such as rash, urticaria, erosion (darkness), rarely the mouth, pharynx (part of the digestive tract between the mouth and esophagus), etc.,
2) If you have a high fever or headache,
3) If you feel nauseous,
3. Other precautions when using this product.
1) Use it according to the prescribed usage and capacity.
2) Do not drink this product.
3) If you drink it incorrectly, follow the pharmacist or doctor's instructions.
4) Children under the age of 6 should not use it without the instructions of a pharmacist or doctor.
4. Caution in saving.
1) Keep it out of reach of children.
2) Avoid direct sunlight and push it (close the lid) in a cool place with as little moisture as possible.
3) Do not change it into other containers to prevent misuse (wrong use) and preserve quality.