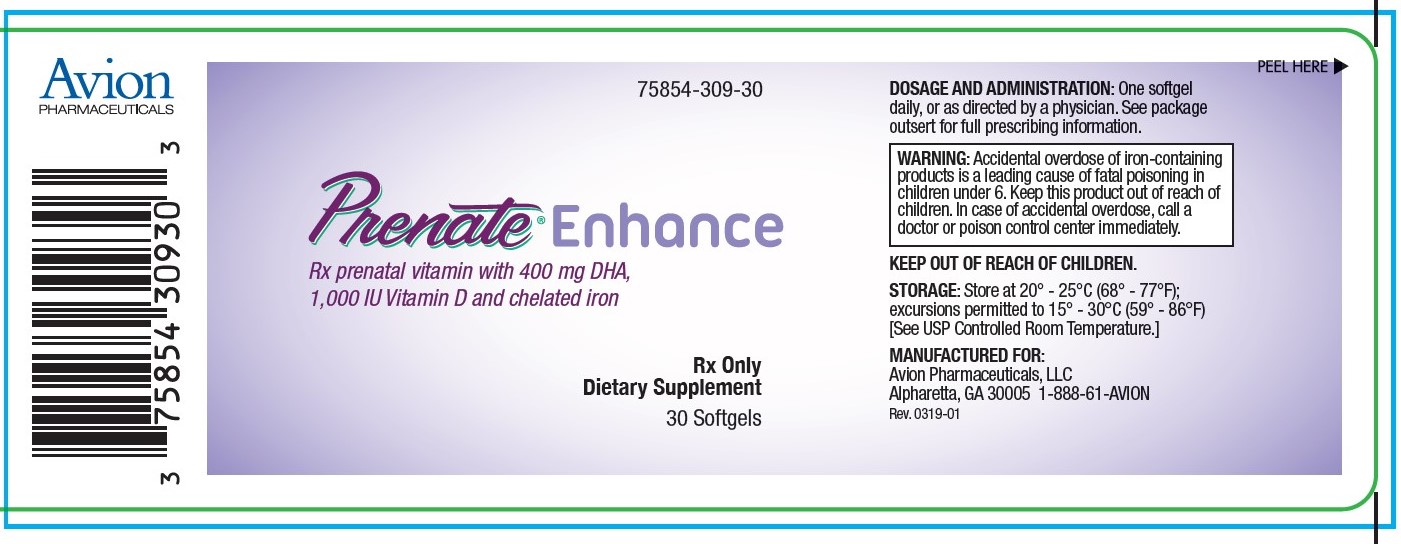
Rx postnatal vitamin with 400 mg DHA, 1,000 IU Vitamin D and chelated iron
Rx Only Dietary Supplement
DESCRIPTION: PRENATE ® Enhance is a prescription prenatal vitamin that contains 400 mg of DHA and advanced calcium. Each dark purple softgel is imprinted with N on one side and blank on the other.
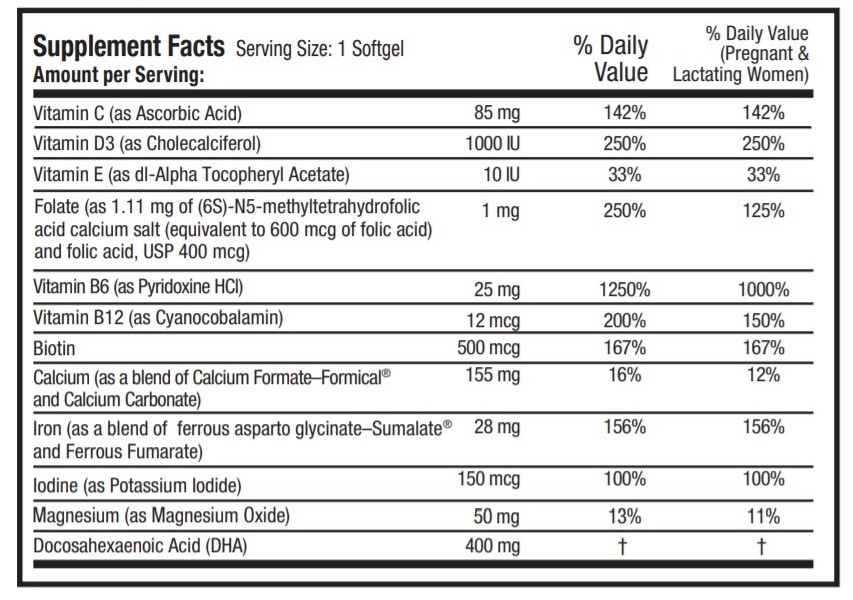
OTHER INGREDIENTS: Gelatin capsule (FD&C Blue # 1, FD&C Red # 40, gelatin, glycerin, purified water, sorbitol, and titanium dioxide), palm shortening, soy lecithin and white beeswax.
INDICATIONS: PRENATE ® Enhance is a multivitamin/multimineral fatty acid dietary supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers. PRENATE ® Enhance can also be beneficial in improving the nutritional status of women prior to conception.
CONTRAINDICATIONS: PRENATE ® Enhance is contraindicated in patients with a known hypersensitivity to any of the ingredients.
WARNING: Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
HOW SUPPLIED: Bottles of 30 softgels (75854-309-30). The listed product number is not a National Drug Code. Instead, Avion Pharmaceuticals has assigned a product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
STORAGE: Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature.]
MANUFACTURED FOR:
Avion Pharmaceuticals, LLC
Atlanta, GA 30005
1-888-61-AVION
Rev. 0319-01
Formical® is a registered trademark of Nephro-Tech 1, LLC, covered by one or more claims of U.S. Patent No. 6,528,542.
Sumalate® is a registered trademark of Albion Laboratories, Inc., covered by one or more claims of U.S. Patent Nos. 5,516,925, 6,716,814, 8,007,846 and 8,425,956.
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE