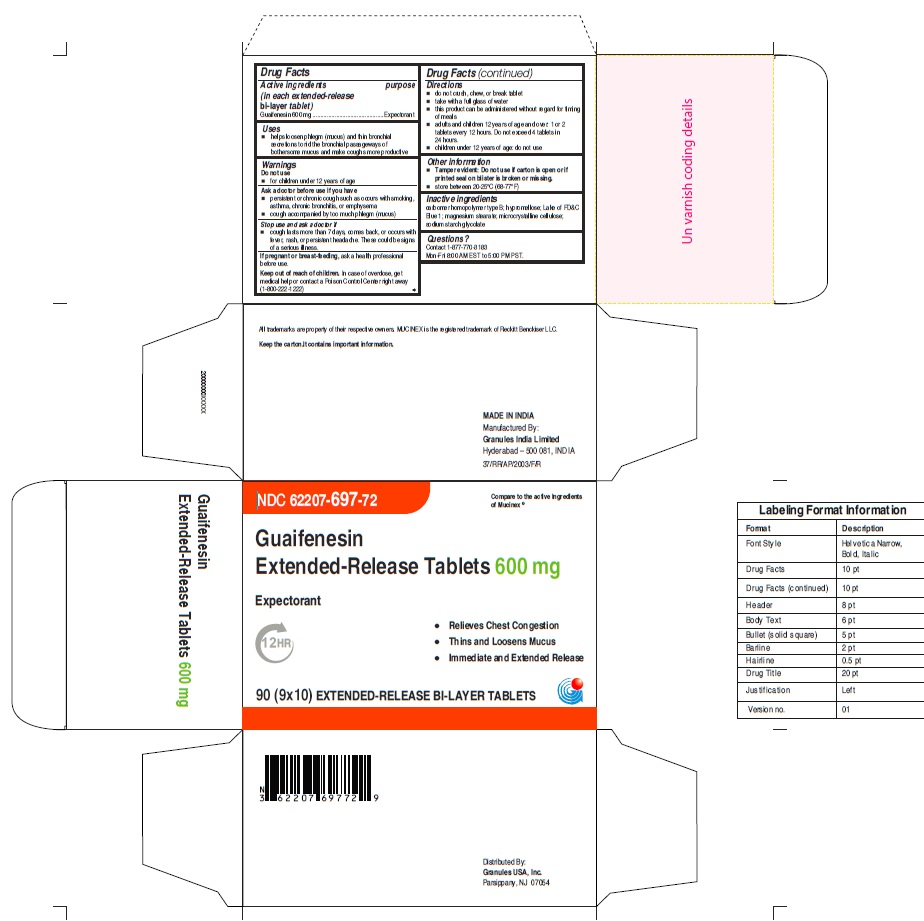
USE(S)
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
ASK A DOCTOR BEFORE USE IF YOU HAVE
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
STOP USE AND ASK DOCTOR IF
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away
(1-800-222-1222)
DIRECTIONS
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours(For 600mg)
- adults and children 12 years of age and over: 1 tablet every 12 hours. Do not exceed 2 tablets in 24 hours.(For 1200mg)
- children under 12 years of age: do not use
OTHER INFORMATION
Tamper evident: Do not use if carton is open or if printed seal on blister is broken or missing.
INACTIVE INGREDIENTS
carbomer homopolymer type B; hypromellose, Lake of FD&C Blue 1, magnesium stearate, microcrystalline cellulose; sodium starch glycolate