Active ingredient (in each tablet)
Caffeine 100 mg
Uses
helps to restore mental alertness or wakefulness when experiencing fatigue or drowsiness.
Warnings
For occasional use only
Do not use
• in children under 12 years of age • as a substitute for sleep
When using this product
Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat. The recommended dose of this product contains about as much caffeine as a cup of coffee.
Stop use and ask a doctor if
fatigue or drowsiness • persists • continues to recur
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center.
Directions
take 1 tablet not more often than every 3 to 4 hours.
• adults and children 12 years of age and over:
Other information
• store at room temperature between 59°-86°F (15°-30°C) • protect from excessive moisture • do not use if tamper-evident seal is broken or missing
Inactive ingredients
calcium stearate, lactose, microcrystalline cellulose, silicon dioxide.
Questions?
1-800-328-5890
Weekdays 8:30-5:00 CST
Package Labeling:
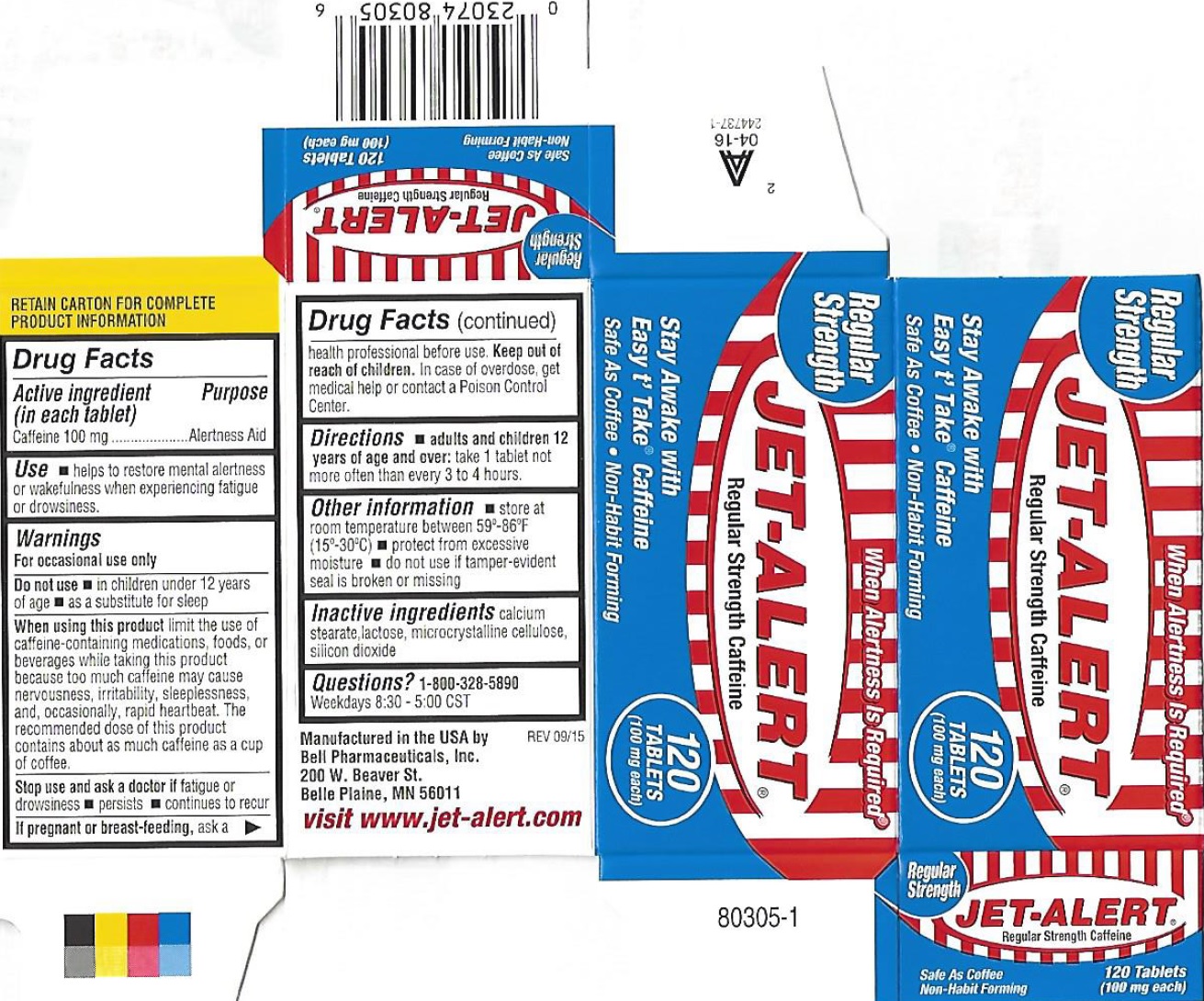

Package Labeling:



