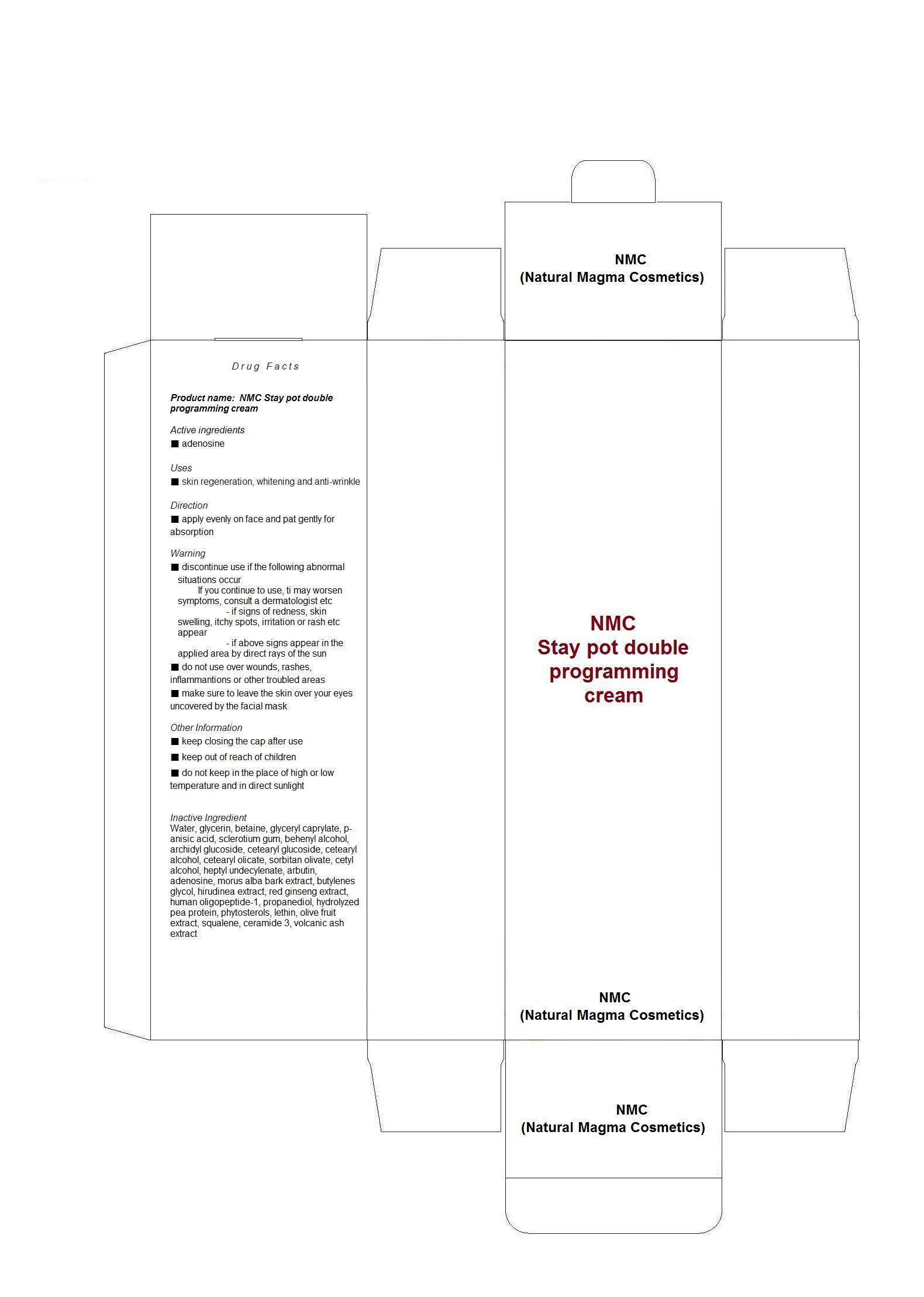
Water, glycerin, betaine, glyceryl caprylate, p-anisic acid, sclerotium gum, behenyl alcohol, archidyl glucoside, cetearyl glucoside, cetearyl alcohol, cetearyl olicate, sorbitan olivate, cetyl alcohol, heptyl undecylenate, arbutin, adenosine, morus alba bark extract, butylenes glycol, hirudinea extract, red ginseng extract, human oligopeptide-1, propanediol, hydrolyzed pea protein, phytosterols, lethin, olive fruit extract, squalene, ceramide 3, volcanic ash extract
■ discontinue use if the following abnormal situations occur
If you continue to use, ti may worsen symptoms, consult a dermatologist etc
- if signs of redness, skin swelling, itchy spots, irritation or rash etc appear
- if above signs appear in the applied area by direct rays of the sun
■ do not use over wounds, rashes, inflammantions or other troubled areas
■ make sure to leave the skin over your eyes uncovered by the facial mask
Other Information
■ keep closing the cap after use
■ keep out of reach of children
■ do not keep in the place of high or low temperature and in direct sunlight