Women who use Medroxyprogesterone Acetate Injectable Suspension, USP may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible.
It is unknown if use of Medroxyprogesterone Acetate Injectable Suspension, USP during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
Medroxyprogesterone Acetate Injectable Suspension, USP should be used as a long-term birth control method (e.g. longer than 2 years) only if other birth control methods are inadequate. (See WARNINGS.)
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
DESCRIPTION
Medroxyprogesterone Acetate Injectable Suspension, USP contains medroxyprogesterone acetate, a derivative of progesterone, as its active ingredient. Medroxyprogesterone Acetate is active by the parenteral and oral routes of administration. It is a white to off-white; odorless crystalline powder that is stable in air and that melts between 200°C and 210°C. It is freely soluble in chloroform, soluble in acetone and dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether, and insoluble in water.
The chemical name for Medroxyprogesterone Acetate is pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6α-).
The structural formula is as follows:
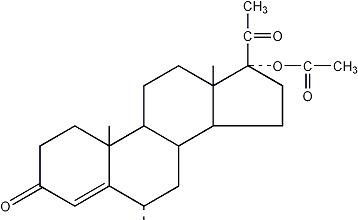
medroxyprogesterone acetate
Medroxyprogesterone Acetate Injectable Suspension, USP for intramuscular (IM) injection is available in vials and prefilled syringes, each containing 1 mL of Medroxyprogesterone Acetate sterile aqueous suspension 150 mg/mL.
Each mL contains:
| Medroxyprogesterone acetate | 150 mg |
| Polyethylene glycol 3350 | 28.9 mg |
| Polysorbate 80 | 2.41 mg |
| Sodium chloride | 8.68 mg |
| Methylparaben | 1.37 mg |
| Propylparaben | 0.150 mg |
| Water for injection | qs |
When necessary, pH is adjusted with sodium hydroxide or hydrochloric acid, or both.
CLINICAL PHARMACOLOGY
Medroxyprogesterone Acetate Injectable Suspension, USP, when administered at the recommended dose to women every 3 months, inhibits the secretion of gonadotropins which, in turn, prevents follicular maturation and ovulation and results in endometrial thinning. These actions produce its contraceptive effect.
Following a single 150 mg IM dose of Medroxyprogesterone Acetate Injectable Suspension, USP, Medroxyprogesterone Acetate concentrations, measured by an extracted radioimmunoassay procedure, increase for approximately 3 weeks to reach peak plasma concentrations of 1 to 7 ng/mL. The levels then decrease exponentially until they become undetectable (<100 pg/mL) between 120 to 200 days following injection. Using an unextracted radioimmunoassay procedure for the assay of Medroxyprogesterone Acetate in serum, the apparent half-life for Medroxyprogesterone Acetate following IM administration of Medroxyprogesterone Acetate Injectable Suspension, USP is approximately 50 days.
Women with lower body weights conceive sooner than women with higher body weights after discontinuing Medroxyprogesterone Acetate Injectable Suspension, USP.
The effect of hepatic and/or renal disease on the pharmacokinetics of Medroxyprogesterone Acetate Injectable Suspension, USP is unknown.
INDICATIONS AND USAGE
Medroxyprogesterone Acetate Injectable Suspension, USP is indicated only for the prevention of pregnancy. The loss of bone mineral density (BMD) in women of all ages and the impact on peak bone mass in adolescents should be considered, along with the decrease in BMD that occurs during pregnancy and/or lactation, in the risk/benefit assessment for women who use Medroxyprogesterone Acetate Injectable Suspension, USP long-term (see WARNINGS.) It is a long-term injectable contraceptive in women when administered at 3-month (13-week) intervals. Dosage does not need to be adjusted for body weight.
In five clinical studies using Medroxyprogesterone Acetate Injectable Suspension, USP, the 12-month failure rate for the group of women treated with Medroxyprogesterone Acetate Injectable Suspension, USP was zero (no pregnancies reported) to 0.7 by Life-Table method. Pregnancy rates with contraceptive measures are typically reported for only the first year of use as shown in Table 1. Except for intrauterine devices (IUD), implants, sterilization, and Medroxyprogesterone Acetate Injectable Suspension, USP, the efficacy of these contraceptive measures depends in part on the reliability of use. The effectiveness of Medroxyprogesterone Acetate Injectable Suspension, USP is dependent on the patient returning every 3 months (13 weeks) for reinjection.
| Method | Lowest Expected | Typical |
|---|---|---|
| Source: Trussell et al1 | ||
| Injectable progestogen | ||
| Medroxyprogesterone | 0.3 | 0.3 |
| Acetate | ||
| Implants | ||
| Norplant (6 capsules) | 0.2† | 0.2† |
| Female sterilization | 0.2 | 0.4 |
| Male sterilization | 0.1 | 0.15 |
| Pill | 3 | |
| Combined | 0.1 | |
| Progestogen only | 0.5 | |
| IUD | 3 | |
| Progestasert | 2 | |
| Copper T 380A | 0.8 | |
| Condom | 2 | 12 |
| Diaphragm | 6 | 18 |
| Cap | 6 | 18 |
| Spermicides | 3 | 21 |
| Sponge | ||
| Parous women | 9 | 28 |
| Nulliparous women | 6 | 18 |
| Periodic abstinence | 1–9 | 20 |
| Withdrawal | 4 | 18 |
| No method | 85 | 85 |
CONTRAINDICATIONS
- Known or suspected pregnancy or as a diagnostic test for pregnancy.
- Undiagnosed vaginal bleeding.
- Known or suspected malignancy of breast.
- Active thrombophlebitis, or current or past history of thromboembolic disorders, or cerebral vascular disease.
- Significant liver disease.
- Known hypersensitivity to Medroxyprogesterone Acetate Injectable Suspension, USP (Medroxyprogesterone Acetate or any of its other ingredients).
WARNINGS
1. Loss of Bone Mineral Density
Use of Medroxyprogesterone Acetate Injectable Suspension, USP reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD) as bone metabolism accommodates to a lower estrogen level. This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of Medroxyprogesterone Acetate Injectable Suspension, USP by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life. In both adults and adolescents, the decrease in BMD appears to be at least partially reversible after Medroxyprogesterone Acetate Injectable Suspension, USP is discontinued and ovarian estrogen production increases. A study to assess the reversibility of loss of BMD in adolescents is ongoing.
Medroxyprogesterone Acetate Injectable Suspension, USP should be used as a long-term birth control method (e.g. longer than 2 years) only if other birth control methods are inadequate. BMD should be evaluated when a woman needs to continue to use Medroxyprogesterone Acetate Injectable Suspension, USP long term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods should be considered in the risk/benefit analysis for the use of Medroxyprogesterone Acetate Injectable Suspension, USP in women with osteoporosis risk factors. Medroxyprogesterone Acetate Injectable Suspension, USP can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids). Although there are no studies addressing whether calcium and Vitamin D may lessen BMD loss in women using Medroxyprogesterone Acetate Injectable Suspension, USP, all patients should have adequate calcium and Vitamin D intake.
BMD Changes in Adult Women
In a controlled, clinical study, adult women using Medroxyprogesterone Acetate Injectable Suspension, USP for up to 5 years showed spine and hip BMD mean decreases of 5–6%, compared to no significant change in BMD in the control group. The decline in BMD was more pronounced during the first two years of use, with smaller declines in subsequent years. Mean changes in lumbar spine BMD of -2.86%, -4.11%, -4.89%, -4.93% and -5.38% after 1, 2, 3, 4, and 5 years, respectively, were observed. Mean decreases in BMD of the total hip and femoral neck were similar.
After stopping use of Medroxyprogesterone Acetate Injectable Suspension, USP (150 mg), there was partial recovery of BMD toward baseline values during the 2-year post-therapy period. Longer duration of treatment was associated with less complete recovery during this 2-year period following the last injection. Table 2 shows the extent of recovery of BMD for women who completed 5 years of treatment.
| Time in Study | Spine | Total Hip | Femoral Neck | |||
|---|---|---|---|---|---|---|
| Medroxy-progesterone Acetate* | Control† | Medroxy-progesterone Acetate* | Control† | Medroxy-progesterone Acetate* | Control† | |
| 5 years | n=33 | n=105 | n=21 | n=65 | n=34 | n=106 |
| -5.38% | 0.43% | -5.16% | 0.19% | -6.12% | -0.27% | |
| 7 years | n=12 | n=60 | n=7 | n=39 | n=13 | n=63 |
| -3.13% | 0.53% | -1.34% | 0.94% | -5.38% | -0.11% | |
BMD Changes in Adolescent Females (12–18 years of age)
Preliminary results from an ongoing, open-label, self-selected, non-randomized clinical study of adolescent females (12–18 years) also showed that Medroxyprogesterone Acetate Injectable Suspension, USP use was associated with a significant decline in BMD from baseline (Table 3). In general, adolescents increase bone density during the period of growth following menarche, as seen in the untreated cohort. However, the two cohorts were not matched at baseline for age, gynecologic age, race, BMD and other factors that influence the rate of acquisition of bone mineral density, with the result that they differed with respect to these demographic factors.
Preliminary data from the small number of adolescents participating in the 2-year post-use observation period demonstrated partial recovery of BMD.
| Duration of Treatment | Medroxyprogesterone Acetate Injectable Suspension, USP (150 mg IM) | Unmatched, Untreated Cohort |
||
|---|---|---|---|---|
| N | Mean % Change | N | Mean % Change | |
| Total Hip BMD | ||||
| Week 60 (1.2 years) | 103 | -2.82 | 171 | 1.32 |
| Week 144 (2.8 years) | 45 | -6.16 | 111 | 1.74 |
| Week 240 (4.6 years) | 9 | -6.92 | 69 | 1.12 |
| Femoral Neck BMD | ||||
| Week 60 | 103 | -3.05 | 171 | 1.87 |
| Week 144 | 45 | -6.01 | 111 | 2.54 |
| Week 240 | 9 | -6.06 | 69 | 1.45 |
| Lumbar Spine BMD | ||||
| Week 60 | 104 | -2.42 | 171 | 3.47 |
| Week 144 | 46 | -2.78 | 111 | 5.41 |
| Week 240 | 9 | -4.17 | 70 | 5.12 |
2. Bleeding Irregularities
Most women using Medroxyprogesterone Acetate Injectable Suspension, USP experience disruption of menstrual bleeding patterns. Altered menstrual bleeding patterns include irregular or unpredictable bleeding or spotting, or rarely, heavy or continuous bleeding. If abnormal bleeding persists or is severe, appropriate investigation should be instituted to rule out the possibility of organic pathology, and appropriate treatment should be instituted when necessary.
As women continue using Medroxyprogesterone Acetate Injectable Suspension, USP, fewer experience irregular bleeding and more experience amenorrhea. By month 12 amenorrhea was reported by 55% of women, and by month 24 amenorrhea was reported by 68% of women using Medroxyprogesterone Acetate Injectable Suspension, USP.2
3. Cancer Risks
Long-term case-controlled surveillance of users of Medroxyprogesterone Acetate Injectable Suspension, USP found slight or no increased overall risk of breast cancer3 and no overall increased risk of ovarian,4 liver,5 or cervical6 cancer and a prolonged, protective effect of reducing the risk of endometrial7 cancer in the population of users.
A pooled analysis14 from two case-control studies, the World Health Organization Study3 and the New Zealand Study13, reported the relative risk (RR) of breast cancer for women who had ever used Medroxyprogesterone Acetate Injectable Suspension, USP as 1.1 (95% confidence interval (CI) 0.97 to 1.4). Overall, there was no increase in risk with increasing duration of use of Medroxyprogesterone Acetate Injectable Suspension, USP. The RR of breast cancer for women of all ages who had initiated use of Medroxyprogesterone Acetate Injectable Suspension, USP within the previous 5 years was estimated to be 2.0 (95% CI 1.5 to 2.8).
The World Health Organization Study3, a component of the pooled analysis14 described above, showed an increased RR of 2.19 (95% CI 1.23 to 3.89) of breast cancer associated with use of Medroxyprogesterone Acetate Injectable Suspension, USP in women whose first exposure to drug was within the previous 4 years and who were under 35 years of age. However, the overall RR for ever-users of Medroxyprogesterone Acetate Injectable Suspension, USP was only 1.2 (95% CI 0.96 to 1.52).
[NOTE: A RR of 1.0 indicates neither an increased nor a decreased risk of cancer associated with the use of the drug, relative to no use of the drug. In the case of the subpopulation with a RR of 2.19, the 95% CI is fairly wide and does not include the value of 1.0, thus inferring an increased risk of breast cancer in the defined subgroup relative to nonusers. The value of 2.19 means that women whose first exposure to drug was within the previous 4 years and who are under 35 years of age have a 2.19 fold (95% CI 1.23 to 3.89-fold) increased risk of breast cancer relative to nonusers. The National Cancer Institute8 reports an average annual incidence rate for breast cancer for US women, all races, age 30 to 34 years of 26.7 per 100,000. A RR of 2.19, thus, increases the possible risk from 26.7 to 58.5 cases per 100,000 women. The attributable risk, thus, is 31.8 per 100,000 women per year.]
A statistically insignificant increase in RR estimates of invasive squamous-cell cervical cancer has been associated with the use of Medroxyprogesterone Acetate Injectable Suspension, USP in women who were first exposed before the age of 35 years (RR 1.22 to 1.28 and 95% CI 0.93 to 1.70). The overall, nonsignificant relative rate of invasive squamous-cell cervical cancer in women who ever used Medroxyprogesterone Acetate Injectable Suspension, USP was estimated to be 1.11 (95% CI 0.96 to 1.29). No trends in risk with duration of use or times since initial or most recent exposure were observed.
4. Thromboembolic Disorders
The physician should be alert to the earliest manifestations of thrombotic disorders (thrombophlebitis, pulmonary embolism, cerebrovascular disorders, and retinal thrombosis). Should any of these occur or be suspected, the drug should not be readministered.
5. Ocular Disorders
Medication should not be readministered pending examination if there is a sudden partial or complete loss of vision or if there is a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, medication should not be readministered.
6. Unexpected Pregnancies
To ensure that Medroxyprogesterone Acetate Injectable Suspension, USP is not administered inadvertently to a pregnant woman, the first injection must be given ONLY during the first 5 days of a normal menstrual period; ONLY within the first 5-days postpartum if not breast-feeding, and if exclusively breast-feeding, ONLY at the sixth postpartum week (see DOSAGE AND ADMINISTRATION).
Neonates from unexpected pregnancies that occur 1 to 2 months after injection of Medroxyprogesterone Acetate Injectable Suspension, USP may be at an increased risk of low birth weight, which, in turn, is associated with an increased risk of neonatal death. The attributable risk is low because such pregnancies are uncommon.9,10
A significant increase in incidence of polysyndactyly and chromosomal anomalies was observed among infants of users of Medroxyprogesterone Acetate Injectable Suspension, USP, the former being most pronounced in women under 30 years of age. The unrelated nature of these defects, the lack of confirmation from other studies, the distant preconceptual exposure to Medroxyprogesterone Acetate Injectable Suspension, USP, and the chance effects due to multiple statistical comparisons, make a causal association unlikely.11
Neonates exposed to Medroxyprogesterone Acetate in utero and followed to adolescence, showed no evidence of any adverse effects on their health including their physical, intellectual, sexual, or social development.
Several reports suggest an association between intrauterine exposure to progestational drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. The risk of hypospadias (five to eight per 1,000 male births in the general population) may be approximately doubled with exposure to these drugs. There are insufficient data to quantify the risk to exposed female fetuses, but because some of these drugs induce mild virilization of the external genitalia of the female fetus and because of the increased association of hypospadias in the male fetus, it is prudent to avoid the use of these drugs during the first trimester of pregnancy.
To ensure that Medroxyprogesterone Acetate Injectable Suspension, USP is not administered inadvertently to a pregnant woman, it is important that the first injection be given only during the first 5 days after the onset of a normal menstrual period within 5 days postpartum if not breast-feeding and if breast-feeding, at the sixth week postpartum (see DOSAGE AND ADMINISTRATION).
7. Ectopic Pregnancy
Health-care providers should be alert to the possibility of an ectopic pregnancy among women using Medroxyprogesterone Acetate Injectable Suspension, USP who become pregnant or complain of severe abdominal pain.
8. Lactation
Detectable amounts of drug have been identified in the milk of mothers receiving Medroxyprogesterone Acetate Injectable Suspension, USP. In nursing mothers treated with Medroxyprogesterone Acetate Injectable Suspension, USP, milk composition, quality, and amount are not adversely affected. Neonates and infants exposed to medroxyprogesterone from breast milk have been studied for developmental and behavioral effects through puberty. No adverse effects have been noted.
9. Anaphylaxis and Anaphylactoid Reaction
Anaphylaxis and anaphylactoid reaction have been reported with the use of Medroxyprogesterone Acetate Injectable Suspension, USP. If an anaphylactic reaction occurs appropriate therapy should be instituted. Serious anaphylactic reactions require emergency medical treatment.
PRECAUTIONS
GENERAL
1. Physical Examination
It is good medical practice for all women to have annual history and physical examinations, including women using Medroxyprogesterone Acetate Injectable Suspension, USP. The physical examination, however, may be deferred until after initiation of Medroxyprogesterone Acetate Injectable Suspension, USP if requested by the woman and judged appropriate by the clinician. The physical examination should include special reference to blood pressure, breasts, abdomen and pelvic organs, including cervical cytology and relevant laboratory tests. In case of undiagnosed, persistent or recurrent abnormal vaginal bleeding, appropriate measures should be conducted to rule out malignancy. Women with a strong family history of breast cancer or who have breast nodules should be monitored with particular care.
2. Fluid Retention
Because progestational drugs may cause some degree of fluid retention, conditions that might be influenced by this condition, such as epilepsy, migraine, asthma, and cardiac or renal dysfunction, require careful observation.
3. Weight Changes
There is a tendency for women to gain weight while on therapy with Medroxyprogesterone Acetate Injectable Suspension, USP. From an initial average body weight of 136 lb, women who completed 1 year of therapy with Medroxyprogesterone Acetate Injectable Suspension, USP gained an average of 5.4 lb. Women who completed 2 years of therapy gained an average of 8.1 lb.
Women who completed 4 years gained an average of 13.8 lb. Women who completed 6 years gained an average of 16.5 lb. Two percent of women withdrew from a large-scale clinical trial because of excessive weight gain.
4. Return of Fertility
Medroxyprogesterone Acetate Injectable Suspension, USP has a prolonged contraceptive effect. In a large US study of women who discontinued use of Medroxyprogesterone Acetate Injectable Suspension, USP to become pregnant, data are available for 61% of them. Based on Life-Table analysis of these data, it is expected that 68% of women who do become pregnant may conceive within 12 months, 83% may conceive within 15 months, and 93% may conceive within 18 months from the last injection. The median time to conception for those who do conceive is 10 months following the last injection with a range of 4 to 31 months, and is unrelated to the duration of use. No data are available for 39% of the patients who discontinued Medroxyprogesterone Acetate Injectable Suspension, USP to become pregnant and who were lost to follow-up or changed their mind.
5. CNS Disorders and Convulsions
Patients who have a history of psychic depression should be carefully observed and the drug not be readministered if the depression recurs.
There have been a few reported cases of convulsions in patients who were treated with Medroxyprogesterone Acetate Injectable Suspension, USP. Association with drug use or pre-existing conditions is not clear.
6. Carbohydrate Metabolism
A decrease in glucose tolerance has been observed in some patients on Medroxyprogesterone Acetate Injectable Suspension, USP treatment. The mechanism of this decrease is obscure. For this reason, diabetic patients should be carefully observed while receiving such therapy.
DRUG INTERACTIONS
Aminoglutethimide administered concomitantly with the Medroxyprogesterone Acetate Injectable Suspension, USP may significantly depress the serum concentrations of medroxyprogesterone acetate.12 Users of Medroxyprogesterone Acetate Injectable Suspension, USP should be warned of the possibility of decreased efficacy with the use of this or any related drugs.
LABORATORY TEST INTERACTIONS
The pathologist should be advised of progestin therapy when relevant specimens are submitted.
The following laboratory tests may be affected by progestins including Medroxyprogesterone Acetate Injectable Suspension, USP:
- (a)
- Plasma and urinary steroid levels are decreased (eg, progesterone, estradiol, pregnanediol, testosterone, cortisol).
- (b)
- Gonadotropin levels are decreased.
- (c)
- Sex-hormone-binding-globulin concentrations are decreased.
- (d)
- Protein-bound iodine and butanol extractable protein-bound iodine may increase.
T3-uptake values may decrease. - (e)
- Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase.
- (f)
- Sulfobromophthalein and other liver function test values may be increased.
- (g)
- The effects of Medroxyprogesterone Acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
PEDIATRIC USE
Medroxyprogesterone Acetate Injectable Suspension, USP is not indicated before menarche. Use of Medroxyprogesterone Acetate Injectable Suspension, USP is associated with significant loss of BMD. This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity. It is unknown if use of Medroxyprogesterone Acetate Injectable Suspension, USP by younger women will reduce peak bone mass and increase the risk of osteoporotic fractures in later life. Other than concerns about loss of BMD, the safety and effectiveness are expected to be the same for postmenarchal adolescents and adult women.
INFORMATION FOR THE PATIENT
See Patient Labeling.
Patient labeling is included with each single-dose vial and prefilled syringe of Medroxyprogesterone Acetate Injectable Suspension, USP to help describe its characteristics to the patient. It is recommended that prospective users be given this labeling and be informed about the risks and benefits associated with the use of Medroxyprogesterone Acetate Injectable Suspension, USP, as compared with other forms of contraception or with no contraception at all. It is recommended that physicians or other health-care providers responsible for those patients advise them at the beginning of treatment that their menstrual cycle may be disrupted and that irregular and unpredictable bleeding or spotting results, and that this usually decreases to the point of amenorrhea as treatment with Medroxyprogesterone Acetate Injectable Suspension, USP continues, without other therapy being required.
ADVERSE REACTIONS
In the largest clinical trial with Medroxyprogesterone Acetate Injectable Suspension, USP, over 3,900 women, who were treated for up to 7 years, reported the following adverse reactions, which may or may not be related to the use of Medroxyprogesterone Acetate Injectable Suspension, USP.
The following adverse reactions were reported by more than 5% of subjects:
- Menstrual irregularities (bleeding or amenorrhea, or both)
- Abdominal pain or discomfort
- Weight changes
- Dizziness
- Headache
- Asthenia (weakness or fatigue)
- Nervousness
Adverse reactions reported by 1% to 5% of subjects using Medroxyprogesterone Acetate Injectable Suspension, USP were:
- Decreased libido or anorgasmia
- Pelvic pain
- Backache
- Breast pain
- Leg cramps
- No hair growth or alopecia
- Depression
- Bloating
- Nausea
- Rash
- Insomnia
- Edema
- Leukorrhea
- Hot flashes
- Acne
- Arthralgia
- Vaginitis
Events reported by fewer than 1% of subjects included: galactorrhea, melasma, chloasma, convulsions, changes in appetite, gastrointestinal disturbances, jaundice, genitourinary infections, vaginal cysts, dyspareunia, paresthesia, chest pain, pulmonary embolus, allergic reactions, anemia, drowsiness, syncope, dyspnea and asthma, tachycardia, fever, excessive sweating and body odor, dry skin, chills, increased libido, excessive thirst, hoarseness, pain at injection site, blood dyscrasia, rectal bleeding, changes in breast size, breast lumps or nipple bleeding, axillary swelling, breast cancer, prevention of lactation, sensation of pregnancy, lack of return to fertility, paralysis, facial palsy, scleroderma, osteoporosis, uterine hyperplasia, cervical cancer, varicose veins, dysmenorrhea, hirsutism, unexpected pregnancy, thrombophlebitis, deep vein thrombosis.
Postmarketing Experience
There have been rare cases of osteoporosis including osteoporotic fractures reported postmarketing in patients taking Medroxyprogesterone Acetate Injectable Suspension, USP. In addition, there have been voluntary reports of anaphylaxis and anaphylactoid reaction associated with the use of Medroxyprogesterone Acetate Injectable Suspension, USP.
DOSAGE AND ADMINISTRATION
Both the 1 mL vial and the 1 mL prefilled syringe of Medroxyprogesterone Acetate Injectable Suspension, USP should be vigorously shaken just before use to ensure that the dose being administered represents a uniform suspension.
The recommended dose is 150 mg of Medroxyprogesterone Acetate Injectable Suspension, USP every 3 months (13 weeks) administered by deep, IM injection in the gluteal or deltoid muscle. To ensure the patient is not pregnant at the time of the first injection, the first injection MUST be given ONLY during the first 5 days of a normal menstrual period; ONLY within the first 5-days postpartum if not breast-feeding; and if exclusively breast-feeding, ONLY at the sixth postpartum week. If the time interval between injections is greater than 13 weeks, the physician should determine that the patient is not pregnant before administering the drug. The efficacy of depends on adherence to the dosage schedule of a Medroxyprogesterone Acetate Injectable Suspension, USP administration.
HOW SUPPLIED
Medroxyprogesterone Acetate Injectable Suspension, USP (Medroxyprogesterone Acetate sterile aqueous suspension 150 mg/mL) is available as:
1 mL vial NDC 54868-5257-0
Store at controlled room temperature 20° to 25°C (68° to 77°F) [see USP].
REFERENCES
- Trussell J, Hatcher RA, Cates W Jr, Stewart FH, Kost K. A guide to interpreting contraceptive efficacy studies. Obstet Gynecol. 1990; 76:558–567.
- Schwallie PC, Assenzo JR. Contraceptive use-efficacy study utilizing Medroxyprogesterone Acetate administered as an intramuscular injection once every 90 days. Fertil Steril. 1973; 24:331–339.
- WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Breast cancer and depot-medroxyprogesterone acetate: a multi-national study. Lancet. 1991; 338:833–838.
- WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Depot-Medroxyprogesterone Acetate (DMPA) and risk of epithelial ovarian cancer. Int J Cancer. 1991; 49:191–195.
- WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Depot-Medroxyprogesterone Acetate (DMPA) and risk of liver cancer. Int J Cancer. 1991; 49:182185.
- WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Depot-Medroxyprogesterone Acetate (DMPA) and risk of invasive squamous-cell cervical cancer. Contraception. 1992; 45:299–312.
- WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Depot-Medroxyprogesterone Acetate (DMPA) and risk of endometrial cancer. Int J Cancer. 1991; 49:186–190.
- Surveillance, Epidemiology, and End Results: Incidence and Mortality Data, 1973–1977. National Cancer Institute Monograph, 57: June 1981. (NIH publication No. 81-2330).
- Gray RH, Pardthaisong T. In Utero exposure to steroid contraceptives and survival during infancy. Am J Epidemiol. 1991; 134:804–811.
- Pardthaisong T, Gray RH. In Utero exposure to steroid contraceptives and outcome of pregnancy. Am J Epidemiol. 1991; 134:795–803.
- Pardthaisong T, Gray RH, McDaniel EB, Chandacham A. Steroid contraceptive use and pregnancy outcome. Teratology. 1988; 38:51–58.
- Van Deijk WA, Biljham GH, Mellink WAM, Meulenberg PMM. Influence of aminoglutethimide on plasma levels of medroxyprogesterone acetate: its correlation with serum cortisol. Cancer Treatment Reports. 1985; 69:1, 85–90.
- Paul C, Skegg DCG, Spears GFS. Depot medroxyprogesterone (Depo-Provera) and risk of breast cancer. Br Med J. 1989; 299:759–762.
- Skegg DCG, Noonan EA, Paul C, Spears GFS, Meirik O, Thomas DB. Depot Medroxyprogesterone Acetate and Breast Cancer: A Pooled Analysis from the World Health Organization and New Zealand Studies. JAMA. 1995; 273(10):799–804.
Medroxyprogesterone Acetate Injectable Suspension, USP
Patient Labeling
Use of Medroxyprogesterone Acetate Injectable Suspension, USP may cause you to lose calcium stored in your bones. The longer you use Medroxyprogesterone Acetate Injectable Suspension, USP the more calcium you are likely to lose. The calcium may not return completely once you stop using Medroxyprogesterone Acetate Injectable Suspension, USP.
Loss of calcium may cause weak, porous bones (osteoporosis) that could increase the risk that your bones might break, especially after menopause. It is not known whether your risk of developing osteoporosis may be greater if you are a teenager when you start to use Medroxyprogesterone Acetate Injectable Suspension, USP.
You should use Medroxyprogesterone Acetate Injectable Suspension, USP long term (for example, more than two years) only if other methods of birth control are not right for you. (See "Risks of Using Medroxyprogesterone Acetate Injectable Suspension, USP")
This product is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Introduction
Every woman who considers using Medroxyprogesterone Acetate Injectable Suspension, USP needs to understand the benefits and risks of this form of birth control and to discuss them with her health-care provider. This leaflet is intended to give you much of the information you will need in order to decide if Medroxyprogesterone Acetate Injectable Suspension, USP is the right choice for you. Your health-care provider will help you to compare Medroxyprogesterone Acetate Injectable Suspension, USP with other contraceptive methods and will answer any questions you have after you have read this information.
Medroxyprogesterone Acetate Injectable Suspension, USP is given as an intramuscular injection (a shot) in the buttock or upper arm once every 3 months (13 weeks). Promptly at the end of the 3-month interval, you will need to return to your health-care provider for your next injection in order to continue your contraceptive protection.
Medroxyprogesterone Acetate Injectable Suspension, USP contains medroxyprogesterone acetate, a chemical similar to (but not the same as) the natural hormone progesterone that is produced by your ovaries during the second half of your menstrual cycle. Medroxyprogesterone Acetate Injectable Suspension, USP acts by preventing your egg cells from ripening. If an egg is not released from the ovaries during your menstrual cycle, it cannot become fertilized by sperm and result in pregnancy. Medroxyprogesterone Acetate Injectable Suspension, USP also causes changes in the lining of your uterus that make it less likely for pregnancy to occur.
Effectiveness of Medroxyprogesterone Acetate Injectable Suspension, USP
To ensure that Medroxyprogesterone Acetate Injectable Suspension, USP is not administered inadvertently to a pregnant woman, the first injection must be given ONLY during the first 5 days of a normal menstrual period; ONLY within the first 5-days postpartum if not breast-feeding, and if exclusively breast-feeding, ONLY at the sixth postpartum week (see Administration of Medroxyprogesterone Acetate Injectable Suspension, USP). The efficacy of Medroxyprogesterone Acetate Injectable Suspension, USP depends on adherence to the recommended dosage schedule.
Medroxyprogesterone Acetate Injectable Suspension, USP is over 99% effective, making it one of the most reliable methods of birth control available. This means that the average annual pregnancy rate is less than one for every 100 women who use Medroxyprogesterone Acetate Injectable Suspension, USP. The effectiveness of most contraceptive methods depends, in part, on how reliably each woman uses the method. The effectiveness of Medroxyprogesterone Acetate Injectable Suspension, USP depends only on the patient returning every 3 months (13 weeks) for her next injection.
The following table shows the percent of women who become pregnant while using different kinds of contraceptive methods. It gives both the lowest expected rate of pregnancy (the rate expected in women who use each method exactly as it should be used) and the typical rate of pregnancy (which includes women who became pregnant because they forgot to use their birth control or because they did not follow the directions exactly).
| Method | Lowest | Typical |
|---|---|---|
| Source: Trussell et al; Obstet Gynecol 1990;76:558–567. | ||
|
||
| Medroxyprogesterone Acetate | 0.3 | 0.3 |
| Implants (Norplant) | 0.2* | 0.2* |
| Female sterilization | 0.2 | 0.4 |
| Male sterilization | 0.1 | 0.15 |
| Oral contraceptives (pill) | — | 3 |
| Combined | 0.1 | — |
| Progestogen only | 0.5 | — |
| IUD | — | 3 |
| Progestasert | 2 | — |
| Copper T 380A | 0.8 | — |
| Condom (without spermicide) | 2 | 12 |
| Diaphragm (with spermicide) | 6 | 18 |
| Cervical cap | 6 | 18 |
| Withdrawal | 4 | 18 |
| Periodic abstinence | 1–9 | 20 |
| Spermicide alone | 3 | 21 |
| Vaginal sponge | — | — |
| used before childbirth | 6 | 18 |
| used after childbirth | 9 | 28 |
| No method | 85 | 85 |
Who Should Not Use Medroxyprogesterone Acetate Injectable Suspension, USP
Certain women should not use Medroxyprogesterone Acetate Injectable Suspension, USP. You should not use Medroxyprogesterone Acetate Injectable Suspension, USP if you have any of the following conditions:
- if you think you might be pregnant
- if you have any vaginal bleeding without a known reason
- if you have had cancer of the breast
- if you have had a stroke
- if you have or have had blood clots (phlebitis) in your legs
- if you have problems with your liver or liver disease
- if you are allergic to Medroxyprogesterone Acetate Injectable Suspension, USP (medroxyprogesterone acetate or any of its other ingredients)
Other Things to Consider Before Choosing Medroxyprogesterone Acetate Injectable Suspension, USP
Before your doctor prescribes Medroxyprogesterone Acetate Injectable Suspension, USP, you will have a physical examination. It is important to tell your doctor or health-care provider if you have any of the following:
- a family history of cancer of the breast
- an abnormal mammogram (breast X-ray), fibrocystic breast disease, breast nodules or lumps, or bleeding from your nipples
- kidney disease
- irregular or scanty menstrual periods
- high blood pressure
- migraine headaches
- asthma
- epilepsy (convulsions or seizures)
- diabetes or a family history of diabetes
- a history of depression
- if you are taking any prescription or over-the-counter medications
This product is intended to prevent pregnancy. It does not protect against transmission of HIV (AIDS) and other sexually transmitted diseases such as chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
Return of Fertility
Because Medroxyprogesterone Acetate Injectable Suspension, USP is a long-acting birth control method, it takes some time after your last injection for its effect to wear off. Based on the results from a large study done in the United States, of those women who stop using Medroxyprogesterone Acetate Injectable Suspension, USP in order to become pregnant, about half of those who become pregnant do so in about 10 months after their last injection; about two-thirds of those who become pregnant do so in about 12 months, about 83% of those who become pregnant do so in about 15 months, and about 93% of those who become pregnant do so in about 18 months after their last injection. The length of time you use Medroxyprogesterone Acetate Injectable Suspension, USP has no effect on how long it takes you to become pregnant after you stop using it.
Risks of Using Medroxyprogesterone Acetate Injectable Suspension, USP
1. Losing Calcium from Your Bones
Medroxyprogesterone Acetate Injectable Suspension, USP use may decrease the amount of calcium in your bones. The longer you are on Medroxyprogesterone Acetate Injectable Suspension, USP the more calcium you may lose. This increases the risk of your bones weakening if you use Medroxyprogesterone Acetate Injectable Suspension, USP continuously for a long time (for more than 2 years). The loss of calcium may increase your risk of osteoporosis and broken bones, particularly after your menopause.
Calcium is generally added to the bones during teenage years. The decrease of calcium in your bones is of most concern if you are a teenager or have the following risk factors:
- -
- bone disease
- -
- anorexia nervosa (an eating disorder)
- -
- a strong family history of osteoporosis
- -
- drug use that can lower the amount of calcium in bones (drugs for epilepsy or steroids), or
- -
- drinking a lot of alcohol or smoking a lot.
If you need a birth control method for more than 2 years, your healthcare provider may ask you to switch to another birth control method or ask you to have a test of your bones before continuing Medroxyprogesterone Acetate Injectable Suspension, USP, especially if you have other risks for weak bones. When Medroxyprogesterone Acetate Injectable Suspension, USP is stopped, the calcium in bones begins to come back. Your healthcare provider may tell you take calcium and Vitamin D as this may lessen the loss of calcium from your bones.
2. Irregular Menstrual Bleeding
The side effect reported most frequently by women who use Medroxyprogesterone Acetate Injectable Suspension, USP for contraception is a change in their normal menstrual cycle. During the first year of using Medroxyprogesterone Acetate Injectable Suspension, USP, you might have one or more of the following changes:
- irregular or unpredictable bleeding or spotting,
- an increase or decrease in menstrual bleeding, or
- no bleeding at all.
Unusually heavy or continuous bleeding, however, is not a usual effect of Medroxyprogesterone Acetate Injectable Suspension, USP and if this happens you should see your health-care provider right away.
With continued use of Medroxyprogesterone Acetate Injectable Suspension, USP, bleeding usually decreases and many women stop having periods completely. In clinical studies of Medroxyprogesterone Acetate Injectable Suspension, USP, 55% of the women studied reported no menstrual bleeding (amenorrhea) after 1 year of use and 68% of the women studied reported no menstrual bleeding after 2 years of use.
The reason that your periods stop is because Medroxyprogesterone Acetate Injectable Suspension, USP causes a resting state in your ovaries. When your ovaries do not release an egg monthly, the regular monthly growth of the lining of your uterus does not occur and, therefore, the bleeding that comes with your normal menstruation does not take place. When you stop using Medroxyprogesterone Acetate Injectable Suspension, USP your menstrual period will usually, in time, return to its normal cycle.
3. Cancer
Studies of women who have used different forms of contraception found that women who used Medroxyprogesterone Acetate Injectable Suspension, USP for contraception had no increased overall risk of developing cancer of the breast, ovary, uterus, cervix, or liver. However, women under 35 years of age whose first exposure to Medroxyprogesterone Acetate Injectable Suspension, USP was within the previous 4 to 5 years may have a slightly increased risk of developing breast cancer similar to that seen with oral contraceptives. You should discuss this with your health-care provider.
4. Unexpected Pregnancy
Because Medroxyprogesterone Acetate Injectable Suspension, USP is such an effective contraceptive method, the risk of unexpected pregnancy for women who get their shots regularly (every 3 months [13 weeks] ) is very low. While there have been reports of an increased risk of low birth weight and neonatal infant death or other health problems in infants conceived close to the time of injection, such pregnancies are uncommon. If you think you may have become pregnant while using Medroxyprogesterone Acetate Injectable Suspension, USP for contraception, see your health-care provider as soon as possible.
5. Allergic Reactions
Severe allergic reactions known as anaphylaxis and anaphylactoid reactions have also been reported in some women using Medroxyprogesterone Acetate Injectable Suspension, USP.
6. Other Risks
Women who use hormone-based contraceptives may have an increased risk of blood clots or stroke. Also, if a contraceptive method fails, there is a possibility that the fertilized egg will begin to develop outside of the uterus (ectopic pregnancy). While these events are rare, you should tell your health-care provider if you have any of the Warning Signals listed in the next section.
Warning Signals
If any of these problems occur following an injection of Medroxyprogesterone Acetate Injectable Suspension, USP, call your healthcare provider immediately:
- Sharp chest pain, coughing up of blood, or sudden shortness of breath (indicating a possible clot in the lung)
- Sudden severe headache or vomiting, dizziness or fainting, problems with your eyesight or speech, weakness, or numbness in an arm or leg (indicating a possible stroke)
- Severe pain or swelling in the calf (indicating a possible clot in the leg)
- Unusually heavy vaginal bleeding
- Severe pain or tenderness in the lower abdominal area
- Persistent pain, pus, or bleeding at the injection site
Side Effects of Medroxyprogesterone Acetate Injectable Suspension, USP
1. Weight Gain
You may experience a weight gain while you are using Medroxyprogesterone Acetate Injectable Suspension, USP. About two-thirds of the women who used Medroxyprogesterone Acetate Injectable Suspension, USP in the clinical trials reported a weight gain of about 5 pounds during the first year of use. You may continue to gain weight after the first year. Women in one large study who used Medroxyprogesterone Acetate Injectable Suspension, USP for 2 years gained an average total of 8.1 pounds over those 2 years, or approximately 4 pounds per year. Women who continued for 4 years gained an average total of 13.8 pounds over those 4 years, or approximately 3.5 pounds per year. Women who continued for 6 years gained an average total of 16.5 pounds over those 6 years, or approximately 2.75 pounds per year.
2. Other Side Effects
In a clinical study of over 3,900 women who used Medroxyprogesterone Acetate Injectable Suspension, USP for up to 7 years, some women reported the following effects that may or may not have been related to their use of Medroxyprogesterone Acetate Injectable Suspension, USP:
| • irregular menstrual bleeding | • swelling of the hands or feet |
| • amenorrhea | • backache |
| • headache | • depression |
| • nervousness | • insomnia |
| • abdominal cramps | • acne |
| • dizziness | • pelvic pain |
| • weakness or fatigue | • no hair growth or excessive hair loss |
| • decreased sexual desire | • rash |
| • leg cramps | • hot flashes |
| • nausea | • joint pain |
| • vaginal discharge or irritation | |
| • breast swelling and tenderness | |
| • bloating |
Other problems were reported by very few of the women in the clinical trials, but some of these could be serious. These include: convulsions, jaundice, urinary tract infections, allergic reactions, fainting, paralysis, osteoporosis, lack of return to fertility, deep vein thrombosis, pulmonary embolus, breast cancer, or cervical cancer. If these or any other problems occur during your use of Medroxyprogesterone Acetate Injectable Suspension, USP, discuss them with your health-care provider.
General Precautions
1. Missed Periods
During the time you are using Medroxyprogesterone Acetate Injectable Suspension, USP for contraception, you may skip a period, or your periods may stop completely. If you have been receiving your injection of Medroxyprogesterone Acetate Injectable Suspension, USP regularly every 3 months (13 weeks), then you are probably not pregnant. However, if you think that you may be pregnant, see your health-care provider.
2. Laboratory Test Interactions
If you are scheduled for any laboratory tests, tell your health-care provider that you are using Medroxyprogesterone Acetate Injectable Suspension, USP for contraception. Certain blood tests are affected by hormones such as Medroxyprogesterone Acetate Injectable Suspension, USP.
3. Drug Interactions
Cytadren (aminoglutethimide) is an anticancer drug that may significantly decrease the effectiveness of Medroxyprogesterone Acetate Injectable Suspension, USP if the two drugs are given during the same time.
4. Nursing Mothers
Although Medroxyprogesterone Acetate Injectable Suspension, USP can be passed to the nursing infant in the breast milk, no harmful effects have been found in these children. Medroxyprogesterone Acetate Injectable Suspension, USP does not prevent the breasts from producing milk, so it can be used by nursing mothers. However, to minimize the amount of Medroxyprogesterone Acetate Injectable Suspension, USP that is passed to the infant in the first weeks after birth, you should wait until 6 weeks after childbirth before you start using Medroxyprogesterone Acetate Injectable Suspension, USP for contraception.
Administration of Medroxyprogesterone Acetate Injectable Suspension, USP
The recommended dose of Medroxyprogesterone Acetate Injectable Suspension, USP is 150 mg every 3 months (13 weeks) given in a single intramuscular injection in the buttock or upper arm. To ensure that you are not pregnant at the time of the first injection, it is essential that the injection be given ONLY during the first 5 days of a normal menstrual period. If used following the delivery of a child, the first injection of Medroxyprogesterone Acetate Injectable Suspension, USP MUST be given within 5 days after childbirth if you are not breast-feeding, or if you are exclusively breast-feeding, the injection MUST be given 6 weeks after childbirth. If you wait longer than 3 months (13 weeks) between injections, or longer than 6 weeks after delivery, your health-care provider should determine that you are not pregnant before giving you your injection of Medroxyprogesterone Acetate Injectable Suspension, USP.

