Active ingredient
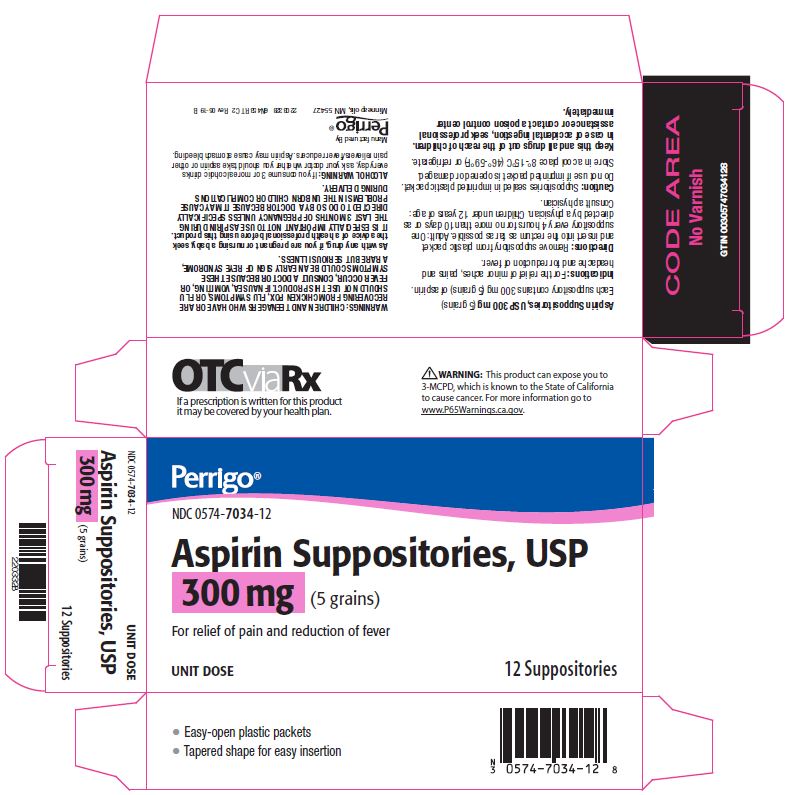
Aspirin Suppositories, USP 300 mg (5 grains)
Each suppository contains 300 mg (5 grains) of aspirin.
OR
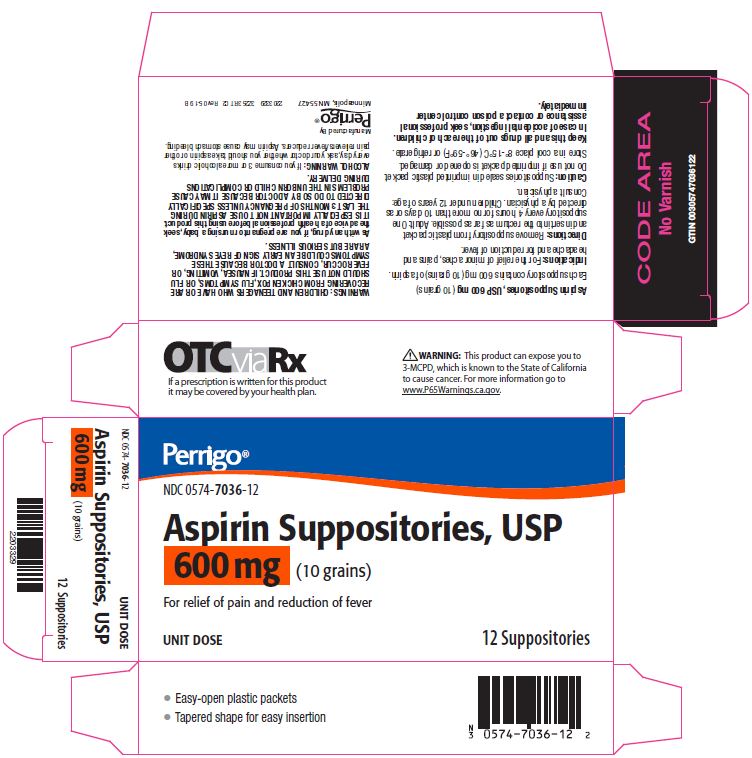
Aspirin Suppositories, USP 600 mg (10 grains)
Each suppository contains 600 mg (10 grains) of aspirin.
Directions
Remove suppository from plastic packet and insert into the rectum as far as possible. Adult: One suppository every 4 hours for no more than 10 days or as directed by a physician. Children under 12 years of age: Consult a physician.
Caution:
Suppositories sealed in imprinted plastic packet. Do not use if imprinted packet is opened or damaged.
Store in a cool place 8° - 15°C (46° - 59°F) or refrigerate.
Keep this and all drugs out of the reach of children.
In case of accidental ingestion, seek professional assistance or contact a poison control center immediately.
Warnings
CHILDREN AND TEENAGERS WHO HAVE OR ARE RECOVERING FROM CHICKEN POX, FLU SYMPTOMS, OR FLU SHOULD NOT USE THIS PRODUCT. IF NAUSEA, VOMITING, OR FEVER OCCUR, CONSULT A DOCTOR BECAUSE THESE SYMPTOMS COULD BE AN EARLY SIGN OF REYE SYNDROME, A RARE BUT SERIOUS ILLNESS.
As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product. IT IS ESPECIALLY IMPORTANT NOT TO USE ASPIRIN DURING THE LAST 3 MONTHS OF PREGNANCY UNLESS SPECIFICALLY DIRECTED TO DO SO BY A DOCTOR BECAUSE IT MAY CAUSE PROBLEMS IN THE UNBORN CHILD OR COMPLICATIONS DURING DELIVERY.
ALCOHOL WARNING:
If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take aspirin or other pain relievers/fever reducers. Aspirin may cause stomach bleeding.
Manufactured by Perrigo®
Minneapolis, MN 55427
Rev 05-19 B