FULL PRESCRIBING INFORMATION
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of XEOMIN and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including lower limb spasticity in children, and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and at lower doses [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
1.1 Chronic Sialorrhea
XEOMIN is indicated for the treatment of chronic sialorrhea in patients 2 years of age and older.
2 DOSAGE AND ADMINISTRATION
2.1 Instructions for Safe Use
The potency Units of XEOMIN for injection are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of XEOMIN cannot be compared to or converted into Units of any other botulinum toxin products assessed with any other specific assay method [see Warnings and Precautions (5.2) and Description (11)]. Reconstituted XEOMIN is intended for intramuscular or intra-salivary gland injection only.
The recommended maximum cumulative dose for any indication should not exceed 400 Units in a treatment session.
2.2 Chronic Sialorrhea
Chronic Sialorrhea in Adult Patients
XEOMIN is injected into the parotid and submandibular glands on both sides (i.e., 4 injection sites per treatment session). The recommended total dose per treatment session is 100 Units. The dose is divided with a ratio of 3:2 between the parotid and submandibular glands (Table 1).
Figure 1: Glands for Injection in Chronic Sialorrhea in Adult Patients
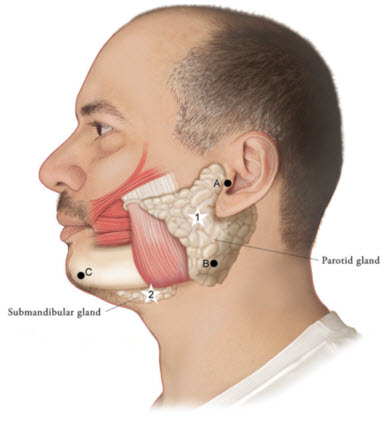
Use the following guidelines if locating salivary glands using anatomic landmarks:
- 1)
- To inject the parotid gland, find the midpoint on the line connecting the tragus and mandible angle (Site A and B, respectively, Figure 1), approximately at the height of the ear lobe. Deliver the injection one finger breadth anterior to this site (Star 1, Figure 1).
- 2)
- To inject the submandibular gland, find the midpoint between the angle of the mandible and the tip of the chin (Site B and C, respectively, Figure 1). Deliver the injection one finger breadth medial to the inferior surface of the mandible at this site (Star 2, Figure 1).
| Gland(s) | Units Per Side | Total |
|---|---|---|
| Parotid gland(s) | 30 Units | 60 Units |
| Submandibular gland(s) | 20 Units | 40 Units |
| Both Glands | 50 Units | 100 Units |
The concentration used in the clinical study after reconstitution was 5 Units/0.1mL. The timing for repeat treatment should be determined based on the actual clinical need of the individual patient, and no sooner than every 16 weeks.
Chronic Sialorrhea in Pediatric Patients
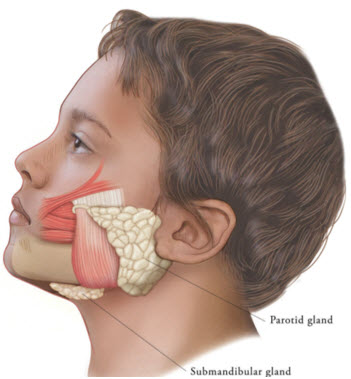
XEOMIN is injected into the parotid and submandibular glands on both sides (i.e., 4 injection sites per treatment session). Ultrasound imaging is recommended to guide needle placement into the salivary glands. The body-weight adjusted dose is divided with a ratio of 3:2 between the parotid and submandibular glands (Table 2). XEOMIN has not been studied in children weighing less than 12 kg [see Clinical Studies (14.1)].
Figure 2: Glands for Injection in Chronic Sialorrhea in Pediatric Patients
| Body weight | Parotid gland, each side | Submandibular gland, each side | Total dose, both glands, both sides | ||
|---|---|---|---|---|---|
| Dose per gland | Volume per injection | Dose per gland | Volume per injection | ||
| 12 kg or more to less than 15 kg | 6 Units | 0.24 mL | 4 Units | 0.16 mL | 20 Units |
| 15 kg or more to less than 19 kg | 9 Units | 0.36 mL | 6 Units | 0.24 mL | 30 Units |
| 19 kg or more to less than 23 kg | 12 Units | 0.48 mL | 8 Units | 0.32 mL | 40 Units |
| 23 kg or more to less than 27 kg | 15 Units | 0.6 mL | 10 Units | 0.4 mL | 50 Units |
| 27 kg or more to less than 30 kg | 18 Units | 0.72 mL | 12 Units | 0.48 mL | 60 Units |
| 30 kg or more | 22.5 Units | 0.9 mL | 15 Units | 0.6 mL | 75 Units |
The concentration used in the clinical study after reconstitution was 2.5 Units/0.1 mL. The timing for repeat treatment should be determined based on the actual clinical need of the individual patient, and no sooner than every 16 weeks.
2.3 Upper Limb Spasticity
Upper Limb Spasticity in Adult Patients
The dosage, frequency, and number of injection sites should be tailored to the individual patient based on the size, number, and location of muscles to be treated, severity of spasticity, presence of local muscle weakness, patient's response to previous treatment, and adverse event history with XEOMIN. The frequency of XEOMIN treatments should be no sooner than every 12 weeks. In patients not previously treated with a botulinum toxin, initial dosing should begin at the low end of the recommended dosing range and titrated as clinically necessary. Most patients in clinical studies were retreated between 12 and 14 weeks.
| Clinical Pattern Muscle | Units (Range) | Number of injection sites per muscle |
|---|---|---|
| Clenched Fist | ||
| Flexor digitorum superficialis | 25 Units-100 Units | 2 |
| Flexor digitorum profundus | 25 Units-100 Units | 2 |
| Flexed Wrist | ||
| Flexor carpi radialis | 25 Units-100 Units | 1-2 |
| Flexor carpi ulnaris | 20 Units-100 Units | 1-2 |
| Flexed Elbow | ||
| Brachioradialis | 25 Units-100 Units | 1-3 |
| Biceps | 50 Units-200 Units | 1-4 |
| Brachialis | 25 Units-100 Units | 1-2 |
| Pronated Forearm | ||
| Pronator quadratus | 10 Units-50 Units | 1 |
| Pronator teres | 25 Units-75 Units | 1-2 |
| Thumb-in-Palm | ||
| Flexor pollicis longus | 10 Units-50 Units | 1 |
| Adductor pollicis | 5 Units-30 Units | 1 |
| Flexor pollicis brevis/Opponens pollicis | 5 Units-30 Units | 1 |
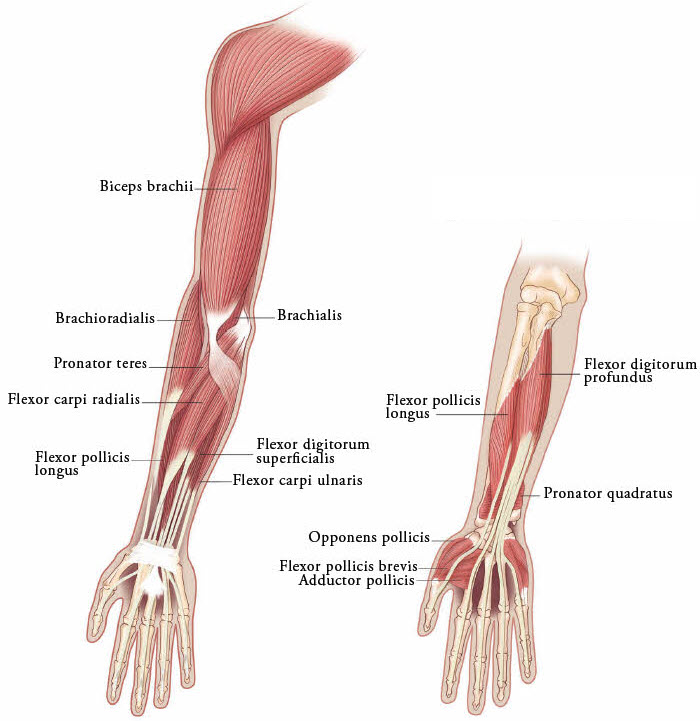
Figure 3: Muscles Involved In Adult Upper Limb Spasticity
Upper Limb Spasticity in Pediatric Patients, Excluding Spasticity Caused by Cerebral Palsy
The exact dosage, frequency, and number of injection sites should be tailored to the individual patient based on size, number and localization of involved muscles; the severity of spasticity; and the presence of local muscle weakness.
The maximum recommended dose is 8 Units/kg, divided among affected muscles, up to a maximum dose of 200 Units per single upper limb. If both upper limbs are treated, total XEOMIN dosage should not exceed 16 Units/kg, up to a maximum of 400 Units.
Based on the selected dose, a reconstituted solution at a concentration between 1.25 Units/0.1 mL and 5 Units/0.1 mL is recommended [see Dosage and Administration (2.7)]. The timing for repeat treatment should be determined based on the clinical need of the patient; the frequency of repeat treatments should be no sooner than every 12 weeks. Most patients in clinical studies were retreated between 12 and 16 weeks.
Table 4 includes the recommended dose ranges for the treatment of the clinical patterns of flexed elbow, flexed wrist, pronated forearm, clenched fist, and thumb-in-palm.
| Clinical Pattern Muscle | Dosage | Number of Injection Sites per Muscle | |
|---|---|---|---|
| Range (Units/kg) | Maximum (Units) |
||
| Flexed Elbow | |||
| Brachioradialis | 1-2 | 50 | 1-2 |
| Biceps | 2-3 | 75 | 1-3 |
| Brachialis | 1-2 | 50 | 1-2 |
| Flexed Wrist | |||
| Flexor carpi radialis | 1 | 25 | 1 |
| Flexor carpi ulnaris | 1 | 25 | 1 |
| Pronated Forearm | |||
| Pronator quadratus | 0.5 | 12.5 | 1 |
| Pronator teres | 1-2 | 50 | 1-2 |
| Clenched Fist | |||
| Flexor digitorum superficialis | 1 | 25 | 1 |
| Flexor digitorum profundus | 1 | 25 | 1 |
| Thumb-in-Palm | |||
| Flexor pollicis longus | 1 | 25 | 1 |
| Adductor pollicis | 0.5 | 12.5 | 1 |
| Flexor pollicis brevis/ opponens pollicis | 0.5 | 12.5 | 1 |
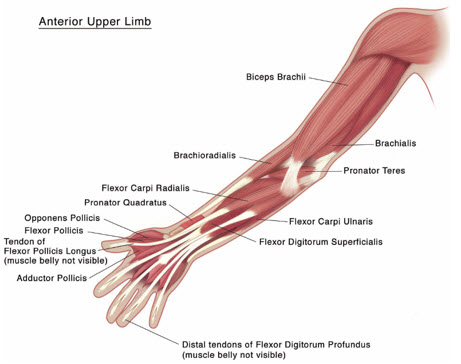
Figure 4: Muscles Injected for Pediatric Upper Limb Spasticity
2.4 Cervical Dystonia
The recommended initial dose of XEOMIN for cervical dystonia is 120 Units. In a placebo-controlled trial utilizing initial XEOMIN doses of 120 Units and 240 Units, no meaningful difference in effectiveness was demonstrated between the doses [see Clinical Studies (14.3)]. In previously treated patients, their past dose, response to treatment, duration of effect, and adverse event history should be taken into consideration when determining the XEOMIN dose.
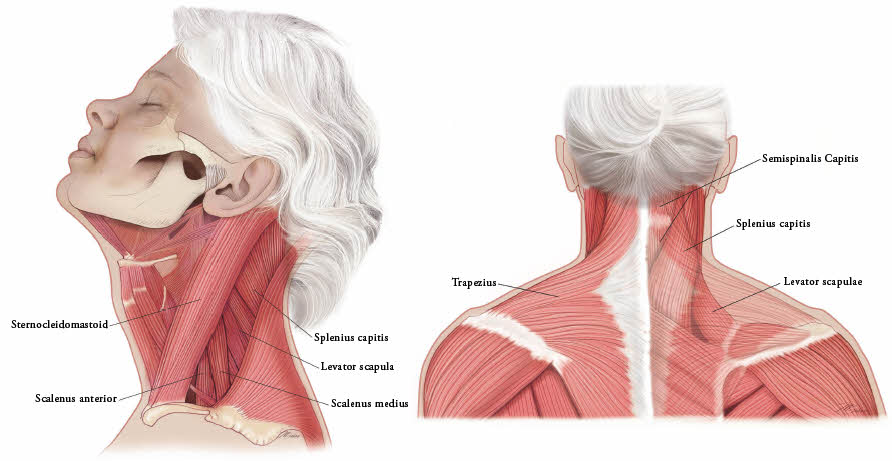
In the treatment of cervical dystonia, XEOMIN is usually injected into the sternocleidomastoid, levator scapulae, splenius capitis, scalenus, and/or the trapezius muscle(s) (see Figure 5). This list is not exhaustive, as any of the muscles responsible for controlling head position may require treatment [see Clinical Studies (14.3)]. The dose and number of injection sites in each treated muscle should be individualized based on the number and location of the muscle(s) to be treated, the degree of spasticity/dystonia, muscle mass, body weight, and response to any previous botulinum toxin injections.
The frequency of XEOMIN repeat treatments should be determined by clinical response, but should generally be no more frequent than every 12 weeks [see Clinical Studies (14.3)].
Figure 5: Muscles Involved in Cervical Dsytonia
2.5 Blepharospasm
In treatment-naïve patients, the recommended initial dose of XEOMIN is 50 Units (25 Units per eye). In patients previously treated with a botulinum toxin A, their past dose, response to treatment, duration of effect, and adverse event history should be taken into consideration when determining the XEOMIN dose.
The total dose of XEOMIN should not exceed 100 Units per treatment session (50 Units per eye).
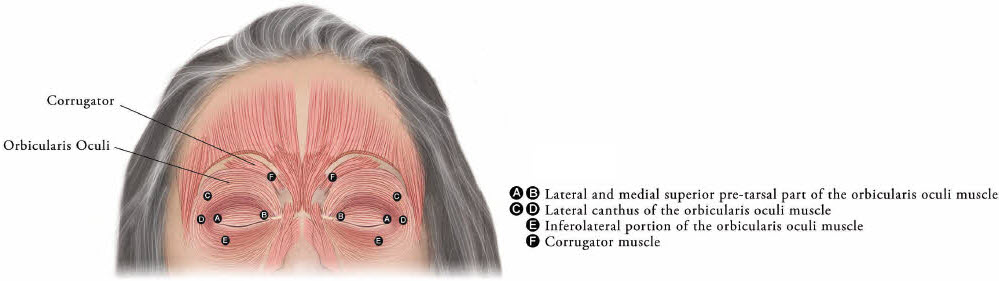
XEOMIN is injected into the lateral and medial orbicularis oculi muscle of the upper lid; lateral canthus and the lateral orbicularis oculi muscle of the lower lid; and the corrugator muscle, if necessary (see Figure 6). The number and location of injections may be changed in response to adverse reactions or based on the patient's response to treatment, but the total dose should not exceed 50 Units per eye.
Figure 6: Injection Sites for Blepharospasm
The frequency of XEOMIN repeat treatments should be determined by clinical response but should generally be no more frequent than every 12 weeks [see Clinical Studies (14.4)].
2.6 Glabellar Lines
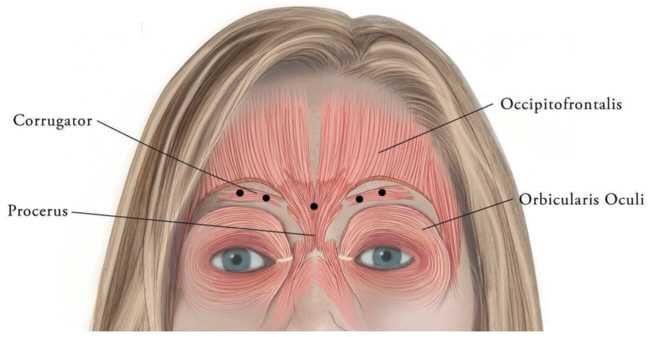
The total recommended XEOMIN dose is 20 Units per treatment session divided into five equal intramuscular injections of 4 Units each. The five injection sites are: two injections in each corrugator muscle and one injection in the procerus muscle.
Retreatment with XEOMIN should be administered no more frequently than every three months.
Figure 7: Injection Sites for Glabellar Lines
2.7 Preparation and Reconstitution Technique
Prior to injection, reconstitute each vial of XEOMIN with sterile, preservative-free 0.9% Sodium Chloride Injection, USP [see Dosage Form and Strengths (3)]. A 20-27 gauge short bevel needle is recommended for reconstitution. Draw up an appropriate amount of preservative-free 0.9% Sodium Chloride Injection, USP into a syringe (see Table 5). Clean the exposed portion of the rubber stopper of the vial with alcohol (70%) prior to insertion of the needle. After vertical insertion of the needle through the rubber stopper, the vacuum will draw the saline into the vial. Gently inject any remaining saline into the vial to avoid foam formation. If the vacuum does not pull the saline into the vial, then XEOMIN must be discarded. Remove the syringe from the vial and mix XEOMIN with the saline by carefully swirling and inverting/flipping the vial – do not shake vigorously. Reconstituted XEOMIN is a clear, colorless solution free of particulate matter. XEOMIN should not be used if the reconstituted solution has a cloudy appearance or contains floccular or particulate matter.
After reconstitution, XEOMIN should be used for only one injection session and for only one patient. Reconstituted XEOMIN solution should be administered within 24 hours after dilution. During this time period, unused reconstituted XEOMIN may be stored in the original container in a refrigerator 2°C -8°C (36°F -46°F) for up to 24 hours until time of use. XEOMIN vials are for single-dose only. Discard any unused portion.
Diluent volumes for reconstitution of XEOMIN are indicated in Table 5.
| Volume of preservative-free 0.9% Sodium Chloride Injection, USP | 50 Unit Vial: Resulting dose in Units per 0.1 mL | 100 Unit Vial: Resulting dose in Units per 0.1 mL | 200 Unit Vial: Resulting dose in Units per 0.1 mL |
|---|---|---|---|
|
|||
| 0.25 mL | 20 Units | - | - |
| 0.5 mL | 10 Units | 20 Units | 40 Units |
| 1 mL | 5 Units | 10 Units | 20 Units |
| 1.25 mL | 4 Units | 8 Units | 16 Units |
| 2 mL | 2.5 Units | 5 Units | 10 Units |
| 2.5 mL | 2 Units | 4 Units | 8 Units |
| 4 mL | 1.25 Units | 2.5 Units | 5 Units |
| 5 mL | 1 Unit | 2 Units | 4 Units |
| 8 mL* | - | 1.25 Units | 2.5 Units |
| 16 mL† | - | - | 1.25 Units |
2.8 Administration
Reconstituted XEOMIN is intended for intramuscular or intra-salivary gland injection only.
If proposed injection sites are marked with a pen, the product must not be injected through the pen marks; otherwise a permanent tattooing effect may occur.
For intramuscular injections, the number of injection sites is dependent upon the size of the muscle to be treated and the volume of reconstituted XEOMIN injected.
XEOMIN should be injected carefully when injected at sites close to sensitive structures, such as the carotid artery, lung apices, and esophagus. Before administering XEOMIN, the physician should be familiar with the patient's anatomy and any anatomic alterations, e.g., due to prior surgical procedures.
Chronic Sialorrhea
Chronic Sialorrhea in Adult Patients
A sterile needle (e.g., 27-30 gauge (0.30-0.40 mm diameter), 12.5 mm length) should be used for intra-salivary gland administration for the treatment of chronic sialorrhea. The injection site should be close to the center of the gland.
The salivary glands can be located using ultrasound imaging or surface anatomical landmarks [see Dosage and Administration (2.2)].
Chronic Sialorrhea in Pediatric Patients
A sterile needle (e.g., 27-30 gauge (0.30-0.40 mm diameter), 12.5 mm length) should be used for intra-salivary gland administration for the treatment of chronic sialorrhea. The injection site should be close to the center of the gland.
Ultrasound guidance is recommended for the localization of the involved salivary glands [see Clinical Studies (14.1)].
Upper Limb Spasticity
Upper Limb Spasticity in Adult Patients
A sterile needle (e.g., 26-gauge (0.45 mm diameter), 37 mm length for superficial muscles; or 22-gauge (0.70 mm diameter), 75 mm length for deeper musculature) should be used in the intramuscular administration in the treatment of upper limb spasticity in adults.
Localization of the involved muscles with electromyographic guidance, nerve stimulation, or ultrasound techniques is recommended.
Upper Limb Spasticity in Pediatric Patients, Excluding Spasticity Caused by Cerebral Palsy
A sterile needle (e.g., 30-gauge (0.30 mm diameter), 25 mm length for superficial muscles; or 27-gauge (0.40 mm diameter), 37 mm length for deeper musculature) should be used in the intramuscular administration in the treatment of upper limb spasticity in pediatric patients.
Localization of the involved muscles with techniques such as electromyographic guidance, nerve stimulation, or ultrasound is recommended.
Cervical Dystonia
A sterile needle (e.g., 26-gauge (0.45 mm diameter), 37 mm length for superficial muscles; or 22-gauge (0.70 mm diameter), 75 mm length for deeper musculature) should be used in the intramuscular administration in the treatment of cervical dystonia.
Localization of the involved muscles with electromyographic guidance, ultrasound, or nerve stimulation techniques may be useful.
3 DOSAGE FORMS AND STRENGTHS
For injection: 50 Units, 100 Units, or 200 Units lyophilized powder in a single-dose vial for reconstitution only with preservative-free 0.9% Sodium Chloride Injection, USP.
4 CONTRAINDICATIONS
XEOMIN is contraindicated in patients with:
- Known hypersensitivity to any botulinum toxin product or to any of the components in the formulation [see Warnings and Precautions (5.3) and Description (11)].
- Infection at the proposed injection site(s) because it could lead to severe local or disseminated infection.
5 WARNINGS AND PRECAUTIONS
5.1 Spread of Toxin Effect
Postmarketing safety data from XEOMIN and other approved botulinum toxins suggest that botulinum toxin effects may, in some cases, be observed beyond the site of local injection. The symptoms are consistent with the mechanism of action of botulinum toxin and may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death related to the spread of toxin effects. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can occur in adults treated for spasticity and other conditions, and particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including lower limb spasticity in children, and in approved indications, symptoms consistent with spread of toxin effect have been reported at doses comparable to or lower than doses used to treat cervical dystonia.
Patients or caregivers should be advised to seek immediate medical care if swallowing, speech, or respiratory disorders occur.
5.2 Lack of Interchangeability between Botulinum Toxin Products
The potency Units of XEOMIN are specific to the preparation and assay method utilized. They are not interchangeable with the other preparations of botulinum toxin products and, therefore, Units of biological activity of XEOMIN cannot be compared to or converted into Units of any other botulinum toxin products assessed with any other specific assay method [see Description (11)].
5.3 Hypersensitivity Reactions
Serious hypersensitivity reactions have been reported with botulinum toxin products. Hypersensitivity reactions include anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea. If serious and/or immediate hypersensitivity reactions occur, discontinue further injection of XEOMIN and institute appropriate medical therapy immediately. The use of XEOMIN in patients with a known hypersensitivity to any botulinum neurotoxin or to any of the excipients (human albumin, sucrose), could lead to a life-threatening allergic reaction [see Contraindications (4)].
5.4 Dysphagia and Breathing Difficulties
Treatment with XEOMIN and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or swallowing. When distant effects occur, additional respiratory muscles may be involved [see Warnings and Precautions (5.1)].
Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several months, and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia, and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised.
Treatment of cervical dystonia with botulinum toxins may weaken neck muscles that serve as accessory muscles of ventilation. This may result in critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these accessory muscles. There have been post-marketing reports of serious breathing difficulties, including respiratory failure, in patients with cervical dystonia treated with botulinum toxin products.
Patients with smaller neck muscle mass and patients who require bilateral injections into the sternocleidomastoid muscles have been reported to be at greater risk of dysphagia. In general, limiting the dose injected into the sternocleidomastoid muscle may decrease the occurrence of dysphagia.
Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Patients with neuromuscular disorders with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junctional disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) may be at increased risk for severe dysphagia and respiratory compromise from typical doses of XEOMIN.
5.5 Corneal Exposure, Corneal Ulceration, and Ectropion in Patients Treated for Blepharospasm
Reduced blinking from injection of botulinum toxin products in the orbicularis muscle can lead to corneal exposure, persistent epithelial defect, and corneal ulceration, especially in patients with VII nerve disorders. As patients with previous eye surgery may have reduced corneal sensation, carefully assess corneal sensation before treatment. Vigorous treatment of any corneal epithelial defect should be employed. This may require protective drops, ointment, therapeutic soft contact lenses, or closure of the eye by patching or other means. Because of its anticholinergic effects, XEOMIN should be used with caution in patients at risk of developing narrow angle glaucoma. To decrease the risk for ectropion, XEOMIN should not be injected into the medial lower eyelid area.
Ecchymosis easily occurs in the soft tissues of the eyelid. Immediate gentle pressure at the injection site can limit the size.
5.6 Risk of Ptosis in Patients Treated for Glabellar Lines
Do not exceed the recommended dosage and frequency of administration of XEOMIN.
In order to reduce the complication of ptosis the following steps should be taken:
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- Corrugator injections should be placed at least 1 cm above the bony supraorbital ridge.
5.7 Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD, or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
6 ADVERSE REACTIONS
The following adverse reactions to XEOMIN are discussed in greater detail in other sections of the labeling:
- Spread of Effects from Toxin [see Warnings and Precautions (5.1)]
- Lack of Interchangeability between Botulinum Toxin Products [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Dysphagia and Breathing Difficulties [see Warnings and Precautions (5.4)]
- Corneal Exposure, Corneal Ulceration, and Ectropion in Patients Treated with XEOMIN for Blepharospasm [see Warnings and Precautions (5.5)]
- Risk of Ptosis in Patients Treated for Glabellar Lines [see Warnings and Precautions (5.6)]
- Human Albumin and Transmission of Viral Diseases [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Chronic Sialorrhea
Chronic Sialorrhea in Adult Patients
Table 6 lists the adverse reactions that occurred in ≥3% of XEOMIN-treated patients in the double-blind, placebo-controlled phase of the study in adult patients with chronic sialorrhea [see Clinical Studies (14.1)]. The most common adverse reactions (≥4%) were tooth extraction, dry mouth, diarrhea, and hypertension. In the controlled portion of this study, 74 patients received 100 Units of XEOMIN, and 36 patients received placebo. XEOMIN-treated patients were 21-80 years old (mean 65 years), and were predominantly male (71%) and White (99.5%).
| Adverse Reaction | XEOMIN 100 Units (N = 74) % | Placebo (N = 36) % |
|---|---|---|
| Tooth extraction | 5 | 0 |
| Dry mouth | 4 | 0 |
| Diarrhea | 4 | 3 |
| Hypertension | 4 | 3 |
| Fall | 3 | 0 |
| Bronchitis | 3 | 0 |
| Dysphonia | 3 | 0 |
| Back pain | 3 | 0 |
| Dry eye | 3 | 0 |
Chronic Sialorrhea in Pediatric Patients
Table 7 lists the adverse reactions that occurred in ≥1% of XEOMIN-treated patients 6-17 years of age in the double-blind, placebo-controlled portion of the study in pediatric patients with chronic sialorrhea [see Clinical Studies (14.1)]. Of the patients 6-17 years of age, 148 patients received a dose of XEOMIN according to body weight, and 72 patients received placebo. Thirty-five patients 2-5 years of age received an open-label dose of XEOMIN according to body weight. XEOMIN-treated patients were 2-17 years of age (mean 10 years), predominately male (63%) and White (100%).
| Adverse Reaction | XEOMIN (6-17 years) (N = 148) % | Placebo (6-17 years) (N = 72) % |
|---|---|---|
| Bronchitis | 1 | 0 |
| Headache | 1 | 0 |
| Nausea/Vomiting | 1 | 0 |
The most frequently reported adverse reaction in patients ages 2-5 years after XEOMIN injections was nasopharyngitis (6%).
In the open-label extension period, 222 patients 2-17 years of age received up to three additional treatments with XEOMIN every 16±2 weeks. The safety profile of XEOMIN during the open-label extension period was similar to that observed in the double-blind phase of the placebo-controlled pediatric chronic sialorrhea study.
Upper Limb Spasticity
Upper Limb Spasticity in Adult Patients
Table 8 lists the adverse reactions that occurred in ≥2% of XEOMIN-treated patients in two placebo-controlled studies in adult patients with upper limb spasticity. Study 1 and Study 2 were both double-blind, placebo-controlled studies, with an open-label extension [see Clinical Studies (14.2)]. In the controlled portion of these studies, 283 patients received ≥120 Units to 400 Units, of which 217 patients received at least 400 Units of XEOMIN, and 182 patients received placebo. XEOMIN-treated patients were 20-79 years of age (mean 56 years), and were predominantly male (58%), and White (84%).
| Adverse Reaction | XEOMIN 400 Units (N = 217) % | Placebo (N = 182) % |
|---|---|---|
| Seizure | 3 | 0 |
| Nasopharyngitis | 2 | 0 |
| Dry mouth | 2 | 1 |
| Upper respiratory tract infection | 2 | 1 |
Upper Limb Spasticity in Pediatric Patients
Table 9 lists the adverse reactions that occurred in ≥2% of XEOMIN-treated patients in Study 1 in pediatric patients 2 years of age and older with upper limb spasticity. In the controlled portion of Study 1, 350 patients were randomized to one of three doses of XEOMIN: 87 received 2 Units/kg per affected upper limb, 87 received 6 Units/kg per affected upper limb, and 176 received 8 Units/kg per affected upper limb [see Clinical Studies (14.2)]. XEOMIN-treated patients were 2 to 17 years of age (mean 7 years), 63% were male, and 90% were White.
No relationship between increased dose and increased occurrence of adverse reactions was observed. The most common adverse reactions (≥3% of XEOMIN-treated patients) at the recommended dose of XEOMIN (8 Units/kg) were nasopharyngitis and bronchitis.
| Adverse Reactions | XEOMIN 2 Units/kg N=87 % | XEOMIN 8 Units/kg N=176 % |
|---|---|---|
|
||
| Infections and infestations | ||
| Nasopharyngitis | 6 | 3 |
| Bronchitis | 2 | 3 |
| Pharyngotonsillitis* | 2 | 2 |
| Upper respiratory tract infection | 2 | 2 |
| Respiratory tract infection viral | 1 | 2 |
| Injury, poisoning and procedural complications | ||
| Fall | 0 | 2 |
| Musculoskeletal and connective tissue disorders | ||
| Pain in extremity | 0 | 2 |
Cervical Dystonia
The data described below reflect exposure to a single intramuscular dose of XEOMIN in a placebo-controlled, Phase 3 trial in patients with cervical dystonia [see Clinical Studies (14.3)]. In this study, 159 patients received XEOMIN (78 were randomized to receive a total dose of 120 Units, and 81 were randomized to receive a total dose of 240 Units). XEOMIN-treated patients were 18 to 79 years old (mean 53 years), and were predominantly female (66%) and Caucasian (91%). At study baseline, approximately 25% had mild, 50% had moderate, and 25% had severe cervical dystonia. Approximately 61% of XEOMIN-treated patients had previously received another botulinum toxin type A product. Table 10 lists adverse reactions that occurred in ≥5% of XEOMIN-treated patients (in any treatment group) and greater than placebo.
| Adverse Reaction | XEOMIN 120 Units (N=77) % | XEOMIN 240 Units (N=82) % | Placebo (N=74) % |
|---|---|---|---|
| Musculoskeletal and connective tissue disorders | 23 | 32 | 11 |
| Neck pain | 7 | 15 | 4 |
| Muscular weakness | 7 | 11 | 1 |
| Musculoskeletal pain | 7 | 4 | 1 |
| Gastrointestinal disorders | 18 | 24 | 4 |
| Dysphagia | 13 | 18 | 3 |
| Nervous system disorders | 16 | 17 | 7 |
| General disorders and administration site conditions | 16 | 11 | 11 |
| Injection site pain | 9 | 4 | 7 |
| Infections and infestations | 14 | 13 | 11 |
| Respiratory, thoracic and mediastinal disorders | 13 | 10 | 3 |
Blepharospasm
Study 1 was a randomized, double-blind, placebo-controlled study that only included treatment-naïve patients [see Clinical Studies (14.3)]. In the controlled portion, 22 patients received XEOMIN 25 Units, 19 patients received 50 Units, and 20 patients received placebo. XEOMIN-treated patients were 23 to 78 years of age (mean 55 years). Fifty-nine percent of the patients were women, 77% were Asian, and 23% White. No patients withdrew prematurely because of an adverse event. Table 11 lists the adverse reactions that occurred in ≥6% of XEOMIN-treated patients and greater than placebo.
| Adverse Reaction | XEOMIN 50 U (N=19) % | Placebo (N=20) % |
|---|---|---|
| Eye disorders | 21 | 10 |
| Eyelid ptosis | 16 | 0 |
Study 2 was a double-blind, placebo-controlled, flexible dose study with an open-label extension (OLEX) period. The study only included patients previously treated with onabotulinumtoxinA (Botox) [see Clinical Studies (14.4)]. In the controlled portion, 74 patients received XEOMIN at a mean dose of approximately 33 Units per eye (minimum 10 Units, maximum 50 Units). XEOMIN-treated patients were 22 to 79 years of age (mean 62 years), predominantly female (65%) and Caucasian (60%). Table 12 lists the adverse reactions that occurred in ≥5% of XEOMIN-treated patients and greater than placebo.
| Adverse Reaction | XEOMIN (N=74) % | Placebo (N=34) % |
|---|---|---|
|
||
| Eye disorders | 38 | 21 |
| Eyelid ptosis | 19 | 9 |
| Dry eye | 16 | 12 |
| Visual impairment* | 12 | 6 |
| Gastrointestinal disorders | 30 | 15 |
| Dry mouth | 16 | 3 |
| Diarrhea | 8 | 0 |
| Infections and infestations | 20 | 15 |
| Nasopharyngitis | 5 | 3 |
| Respiratory tract infection | 5 | 3 |
| Nervous system disorders | 14 | 9 |
| Headache | 7 | 3 |
| General disorders and administration site conditions | 11 | 9 |
| Respiratory, thoracic and mediastinal disorders | 11 | 3 |
| Dyspnea | 5 | 3 |
Glabellar Lines
In three placebo-controlled trials in 803 subjects with glabellar lines, 535 subjects received a single dose of 20 Units XEOMIN and 268 subjects received placebo. XEOMIN-treated subjects were 24 to 74 years old, and were predominantly female (88%). The most frequent adverse reactions in XEOMIN-treated subjects were: headache (5%), facial paresis (0.7%), injection site hematoma (0.6%) and eyelid edema (0.4%). Four serious adverse events occurred in two placebo-treated subjects. Six XEOMIN treated subjects experienced six serious adverse events. All serious adverse events were assessed as unrelated to study drug.
The adverse reactions below reflect exposure to XEOMIN with glabellar lines in placebo-controlled studies. Adverse reactions are adverse events in which there is some basis to believe there is a causal relationship between the drug and the occurrence of the adverse event.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
| Adverse Reaction | XEOMIN (N=535) % | Placebo (N=268) % |
|---|---|---|
| Nervous system disorders | 6 | 2 |
| Headache | 5 | 2 |
| Facial paresis (brow ptosis) | 0.7 | 0 |
| General disorders and administration site conditions | 0.9 | 0.7 |
| Injection site hematoma | 0.6 | 0 |
| Injection site pain | 0.2 | 0 |
| Facial pain | 0.2 | 0 |
| Injection site swelling | 0 | 0.4 |
| Sensation of pressure | 0 | 0.4 |
| Eye disorders | 0.9 | 0 |
| Eyelid edema | 0.4 | 0 |
| Blepharospasm | 0.2 | 0 |
| Eye disorder | 0.2 | 0 |
| Eyelid ptosis | 0.2 | 0 |
In open-label, multiple-dose trials, adverse reactions were reported for 105 of the 800 subjects (13%). Headache was the most common adverse reaction, reported in 7% of subjects, followed by injection site hematoma (1%). Adverse reactions reported in less than 1% of subjects were: facial paresis (brow ptosis), muscle disorder (elevation of eyebrow), injection site pain, and eyelid edema.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other botulinumtoxinA products may be misleading.
Of the 2649 patients treated with XEOMIN in clinical trials [see Clinical Studies (14)], 9 (0.3%) patients were positive for neutralizing antibodies after treatment whose antibody status at baseline was unknown and 4 (0.2%) additional patients developed neutralizing antibodies after treatment. No patients demonstrated a secondary lack of treatment response due to neutralizing antibodies.
Chronic Sialorrhea
Chronic Sialorrhea in Adult Patients
Of the 180 patients treated with XEOMIN in the main phase and extension period of the adult chronic sialorrhea clinical trial [see Clinical Studies (14.1)], 1 (0.6%) patient was positive for neutralizing antibodies after treatment. The patient had an antibody status unknown at baseline, and had not received a botulinum toxin treatment in the 12 months prior to enrollment in the study. No patients demonstrated a secondary lack of treatment response due to neutralizing antibodies.
Chronic Sialorrhea in Pediatric Patients
Of the 252 patients treated with XEOMIN in the main phase and open-label extension period of the pediatric chronic sialorrhea clinical trial [see Clinical Studies (14.1)], antibody measurements were only performed in patients with body weight of 30 kg or more, resulting in 80 patients tested for antibodies at baseline. Three patients tested positive for neutralizing antibodies at baseline and remained positive at the end of the study. No additional patients developed neutralizing antibodies, and none of the patients demonstrated a secondary lack of treatment response.
Upper Limb Spasticity
Upper Limb Spasticity in Adult Patients
Of the 456 patients treated with XEOMIN in the main phase and open-label extension period of the adult upper limb spasticity clinical trials (Study 1 and Study 2) [see Clinical Studies (14.2)], 4 patients were positive for neutralizing antibodies at baseline, and 2 (0.4%) additional patients (with unknown antibody status at baseline) were positive after treatment. Both patients had not received a botulinum toxin treatment in the 12 months prior to enrollment in the studies. No patients demonstrated a secondary lack of treatment response due to neutralizing antibodies.
Upper Limb Spasticity in Pediatric Patients
Of the 907 patients treated with XEOMIN in clinical trials for treatment of pediatric spasticity [see Clinical Studies (14.2)], 7 patients were positive for neutralizing antibodies at baseline, and 4 (0.4%) additional patients (with unknown antibody status at baseline) were positive after treatment. All of these patients were treated with onabotulinumtoxinA and/or abobotulinumtoxinA prior to enrollment in the study. Patients who had never received a botulinum toxin treatment did not develop neutralizing antibodies after being treated with XEOMIN. Antibody measurements were not performed in patients with <21 kg body weight. No patients demonstrated a secondary lack of treatment response due to neutralizing antibodies.
Cervical Dystonia
Of the 227 patients treated with XEOMIN in the main phase and open-label extension period of the cervical dystonia clinical trial [see Clinical Studies (14.3)], 5 patients were positive for neutralizing antibodies at baseline, 1 (0.4%) patient (with unknown antibody status at baseline) was positive after treatment, and 4 (1.8%) additional patients developed neutralizing antibodies after treatment. All of these patients were pre-treated with onabotulinumtoxinA and/or abobotulinumtoxinA prior to enrollment in the study. No patients demonstrated a secondary lack of treatment response due to neutralizing antibodies.
Blepharospasm
Of the 163 patients treated with XEOMIN in the main phase and open-label extension period of the blepharospasm clinical trials (Study 1 and Study 2) [see Clinical Studies (14.4)], 1 (0.6%) patient (with unknown antibody status at baseline) was positive for neutralizing antibodies after treatment. The patient had not received a botulinum toxin treatment in the 12 months prior to enrollment in the studies. No patients demonstrated a secondary lack of treatment response due to neutralizing antibodies.
Glabellar Frown Lines
Of the 464 patients treated with XEOMIN in the main phase and open-label extension period of the glabellar frown lines clinical trials (GL-1 and GL-2) [see Clinical Studies (14.5)], no patients developed neutralizing antibodies after treatment. No patients demonstrated a secondary lack of treatment response due to neutralizing antibodies.
6.3 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of XEOMIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: allergic dermatitis, dysarthria, dysphagia, eye swelling, eyelid edema, flu-like symptoms, herpes zoster, hypersensitivity, injection site pain, injection site reaction, localized allergic reactions (e.g., swelling, edema, erythema, pruritus or rash), muscle spasm, muscular weakness, myalgia, nausea, and persistent dry mouth (> 110 days).
7 DRUG INTERACTIONS
7.1 Aminoglycosides and Other Agents Interfering with Neuromuscular Transmission
Co-administration of XEOMIN and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., tubocurarine-type muscle relaxants) should only be performed with caution as these agents may potentiate the effect of the toxin.
7.2 Anticholinergic Drugs
Use of anticholinergic drugs after administration of XEOMIN may potentiate systemic anticholinergic effects.
7.3 Other Botulinum Neurotoxin Products
The effect of administering different botulinum toxin products at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of XEOMIN in pregnant women. XEOMIN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. XEOMIN was embryotoxic in rats and increased abortions in rabbits when given at doses higher than the maximum recommended human dose (MRHD) for cervical dystonia (120 Units), on a body weight basis.
In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
When XEOMIN was administered intramuscularly to pregnant rats during organogenesis (3 Units/kg, 10 Units/kg, or 30 Units/kg on gestational days [GDs] 6, 12, and 19; or 7 Units/kg on GDs 6 to 19; or 2 Units/kg, 6 Units/kg, or 18 Units/kg on GDs 6, 9, 12, 16, and 19), decreases in fetal body weight and skeletal ossification were observed at doses that were also maternally toxic. The no-effect level for embryotoxicity in rats was 6 Units/kg (3 times the MRHD for cervical dystonia on a body weight basis). Intramuscular administration to pregnant rabbits during organogenesis (1.25 Units/kg, 2.5 Units/kg, or 5.0 Units/kg on GDs 6, 18, and 28) resulted in an increased rate of abortion at the highest dose, which was also maternally toxic. In rabbits, the no-effect level for increased abortion was 2.5 Units/kg (similar to the MRHD for cervical dystonia on a body weight basis).
8.2 Lactation
Risk Summary
There are no data on the presence of XEOMIN in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XEOMIN and any potential adverse effects on the breastfed infant from XEOMIN or from the underlying maternal conditions.
8.4 Pediatric Use
Safety and effectiveness of XEOMIN in patients less than 18 years of age have not been established for lower limb spasticity, cervical dystonia, blepharospasm, or glabellar frown lines [see Warnings and Precautions (5.1)].
Chronic Sialorrhea in Pediatric Patients
The safety and effectiveness of XEOMIN have been established by evidence from an adequate and well-controlled study of XEOMIN in patients 6 to 17 years of age with chronic sialorrhea [See Clinical Studies (14.1)]. Use of XEOMIN in patients 2 to 5 years of age is supported by the findings of efficacy and safety in patients 6 years and older with chronic sialorrhea, and by safety data in patients 2 to 5 years of age. Safety and effectiveness in pediatric patients below the age of 2 years have not been established [see Warnings and Precautions (5.1)].
Upper Limb Spasticity in Pediatric Patients, Excluding Spasticity Caused by Cerebral Palsy
Safety and effectiveness have been established in pediatric patients 2 to 17 years of age [see Warnings and Precautions (5.1), Adverse Reactions (6.1), and Clinical Studies (14.2)]. The safety and effectiveness of XEOMIN have been established by evidence from adequate and well-controlled studies of XEOMIN in patients 2 to 17 years of age with upper limb spasticity. A pediatric assessment for XEOMIN demonstrates that XEOMIN is safe and effective in another pediatric population. However, XEOMIN is not approved for such patient population due to marketing exclusivity for another botulinum toxin. Safety and effectiveness in pediatric patients below the age of 2 years have not been established [see Warnings and Precautions (5.1)].
Juvenile Animal Toxicity Data
In a study in which juvenile rats received intramuscular injections of Xeomin (0, 5, 10, or 30 Units/kg) every other week from postnatal day 21 for 10 weeks, decreased limb use, decreased body weight gain, skeletal muscle atrophy, and decreased bone growth and density were observed at all doses. Male reproductive organ histopathology (atrophy of the germinal epithelium of the testis, associated with hypospermia) was observed at the mid and high doses, and mating behavior was impaired at the high dose. A no-effect dose for adverse effects on development in juvenile animals was not established. The lowest dose tested (5 Units/kg) is less than the human dose of 400 Units on a body weight (kg) basis.
8.5 Geriatric Use
Chronic Sialorrhea
Of the total number of 184 patients in the placebo-controlled study in chronic sialorrhea in adult patients [see Clinical Studies (14.1)], 107 were 65 years of age and over (46 treated with XEOMIN 100 Units, 44 treated with XEOMIN 75 Units, and 17 received placebo). No differences in safety or effectiveness were observed between older and younger patients. Other clinical studies have not identified differences in responses between older and younger patients, but increased sensitivity in older patients cannot be ruled out.
Upper Limb Spasticity
Of the total number of 283 patients in the placebo-controlled studies in upper limb spasticity in adult patients [see Clinical Studies (14.2)], 118 were 65 years of age and over (70 treated with XEOMIN and 48 received placebo), which included 12 patients 75 years of age and over (7 treated with XEOMIN and 5 received placebo). No overall differences in safety or effectiveness were observed between older and younger adult patients. Other clinical studies have not identified differences in responses between older and younger adult patients, but increased sensitivity in older patients cannot be ruled out.
Cervical Dystonia
Of the total number of 233 patients in the placebo-controlled study in cervical dystonia [see Clinical Studies (14.3)], 29 were 65 years of age and over (19 treated with XEOMIN and 10 received placebo). Of these, ten XEOMIN-treated patients and four placebo-treated patients experienced an adverse event. For patients 65 years of age and over treated with XEOMIN, the most common adverse events were dysphagia (21%) and asthenia (11%).
Blepharospasm
Of the total number of 169 patients in the placebo-controlled studies in blepharospasm [see Clinical Studies (14.4)], 61 were 65 years of age and over (45 treated with XEOMIN and 16 received placebo). No overall difference in effectiveness was observed between older and younger patients.
Glabellar Lines
There are limited clinical data with XEOMIN in subjects 65 years of age and over in clinical studies with glabellar lines. Of the total number of 547 subjects in the placebo-controlled clinical studies [see Clinical Studies (14.5)], 21 subjects were 65 years of age and over. Efficacy was observed in 20% (3/15) of XEOMIN subjects 65 years of age and over. For the entire safety database of geriatric subjects, there was no increase in the incidence of adverse events related to treatment with XEOMIN.
10 OVERDOSAGE
Excessive doses of XEOMIN may be expected to produce neuromuscular weakness with a variety of symptoms, particularly when treated intramuscularly. Respiratory support may be required where excessive doses cause paralysis of the respiratory muscles. In the event of overdose, the patient should be medically monitored for symptoms of excessive muscle weakness or muscle paralysis [see Warnings and Precautions (5.1, 5.4)]. Symptomatic treatment may be necessary.
Symptoms of overdose are not likely to be present immediately following injection. Should accidental injection or oral ingestion occur, the person should be medically supervised for several weeks for signs and symptoms of excessive muscle weakness or paralysis.
There is no significant information regarding overdose from clinical studies of XEOMIN.
In the event of overdose, antitoxin raised against botulinum toxin is available from the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. However, the antitoxin will not reverse any botulinum toxin-induced effects already apparent by the time of antitoxin administration. In the event of suspected or actual cases of botulinum toxin poisoning, please contact your local or state Health Department to process a request for antitoxin through the CDC. If you do not receive a response within 30 minutes, please contact the CDC directly at 770-488-7100. More information can be obtained at http://www.cdc.gov/ncidod/srp/drugs/formulary.html#1a.
11 DESCRIPTION
The active ingredient of XEOMIN is botulinum toxin type A produced from fermentation of Hall strain Clostridium botulinum serotype A. The botulinum toxin complex is purified from the culture supernatant and then the active ingredient is separated from the proteins (hemagglutinins and non-hemagglutinins) through a series of steps yielding the active neurotoxin with molecular weight of 150 kDa, without accessory proteins. XEOMIN is a sterile white to off-white lyophilized powder intended for intramuscular or intra-salivary gland injection after reconstitution with preservative-free 0.9% Sodium Chloride Injection, USP [see Dosage Forms and Strengths (3)]. One vial of XEOMIN contains 50 Units, 100 Units, or 200 Units of incobotulinumtoxinA, human albumin (1 mg), and sucrose (4.7 mg).
The primary release procedure for XEOMIN uses a cell-based potency assay to determine the potency relative to a reference standard. One Unit corresponds to the median intraperitoneal lethal dose (LD50) in mice. As the method for conducting the assay is specific to XEOMIN, Units of biological activity of XEOMIN cannot be converted into Units of any other botulinum toxin assessed with other specific assays.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
XEOMIN blocks cholinergic transmission at the neuromuscular and salivary neuroglandular junction by inhibiting the release of acetylcholine from peripheral cholinergic nerve endings. This inhibition occurs according to the following sequence: neurotoxin binding to cholinergic nerve terminals, internalization of the neurotoxin into the nerve terminal, translocation of the light-chain part of the molecule into the cytosol of the nerve terminal, and enzymatic cleavage of SNAP25, a presynaptic target protein essential for the release of acetylcholine. In both muscles and glands, impulse transmission is re-established by the formation of new nerve endings.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Impairment of Fertility
In a fertility and early embryonic development study in rabbits, males and females were dosed with XEOMIN (1.25 Units/kg, 2.5 Units/kg, or 3.5 Units/kg) intramuscularly every two weeks for 5 and 3 doses, respectively, beginning 2 weeks prior to mating. No effects on mating or fertility were observed. The highest dose tested is approximately twice the maximum recommended human dose for cervical dystonia (120 Units) on a body weight basis.
14 CLINICAL STUDIES
14.1 Chronic Sialorrhea
Chronic Sialorrhea in Adult Patients
The efficacy and safety of XEOMIN for the treatment of chronic sialorrhea in adult patients were evaluated in a double-blind, placebo-controlled clinical trial that enrolled a total of 184 patients with chronic sialorrhea resulting from Parkinson's disease, atypical parkinsonism, stroke, or traumatic brain injury, that was present for at least three months. Patients with a history of aspiration pneumonia, amyotrophic lateral sclerosis, salivary gland or duct malformation, and gastroesophageal reflux disease were excluded. The study consisted of a 16-week main phase, followed by an extension period of dose-blinded treatment with XEOMIN.
In the main phase, a fixed total dose of XEOMIN (100 Units or 75 Units) or placebo was administered into the parotid and submandibular salivary glands in a 3:2 dose ratio. The co-primary efficacy variables were the change in unstimulated Salivary Flow Rate (uSFR, Table 14) and the change in Global Impression of Change Scale (GICS, Table 15) at Week 4 post-injection. A total of 173 treated patients completed the main phase of the study. For both the uSFR and GICS, XEOMIN 100 Units was significantly better than placebo (see Table 14 and Table 15). XEOMIN 75 Units was not significantly better than placebo.
| XEOMIN 100 Units | Placebo | |
|---|---|---|
| N=73 | N=36 | |
|
||
| Week 4* | -0.13 | -0.04 |
| Week 8 | -0.13 | -0.02 |
| Week 12 | -0.12 | -0.03 |
| Week 16 | -0.11 | -0.01 |
| XEOMIN 100 Units | Placebo | |
|---|---|---|
| N=74 | N=36 | |
|
||
| Week 4* | 1.25 | 0.67 |
| Week 8 | 1.30 | 0.47 |
| Week 12 | 1.21 | 0.56 |
| Week 16 | 0.93 | 0.41 |
In the extension period, patients received up to three additional treatments with XEOMIN 100 Units or 75 Units every 16±2 weeks, for a total exposure duration of up to 64 weeks. Patients had periodic dental examinations to monitor for changes in dentition and oral mucosa. A total of 151 patients completed the extension period.
Chronic Sialorrhea in Pediatric Patients
The efficacy and safety of XEOMIN for the treatment of chronic sialorrhea in pediatric patients were evaluated in a prospective, randomized, double-blind, placebo-controlled (ages 6-17 years), parallel-group, multicenter trial that enrolled and treated a total of 216 pediatric patients 6-17 years of age with chronic sialorrhea associated with cerebral palsy, other genetic or congenital disorders, or traumatic brain injury. An additional 35 patients 2-5 years of age were treated with open-label XEOMIN in that study. The study consisted of a 16-week main phase, followed by an open-label extension period of treatment with XEOMIN where patients could receive up to 3 additional treatments with XEOMIN every 16 ± 2 weeks, for a total exposure duration of up to 64 weeks (222 patients completed the extension period).
In the main phase, patients 6-17 years of age were administered a total dose of XEOMIN according to body weight (up to 75 Units), or placebo, into the parotid and submandibular glands in a 3:2 dose ratio, using ultrasound guidance. Patients 2-5 years of age all received open-label treatment with XEOMIN, according to body weight, using ultrasound guidance. Patients with a body weight <12 kg were excluded.
The primary efficacy analysis was conducted in the 6-17 years of age patient group. The co-primary endpoints were the change in unstimulated Salivary Flow Rate (uSFR, Table 16) and carer's Global Impression of Change Scale (GICS, Table 17) at Week 4 post-injection.
For both the uSFR and GICS, XEOMIN was statistically significantly better than placebo (see Table 16 and Table 17).
| XEOMIN (6-17 years) N = 148 | Placebo (6-17 years) N=72 |
|
|---|---|---|
|
||
| Week 4* | -0.14 | -0.07 |
| Week 8 | -0.16 | -0.07 |
| Week 12 | -0.16 | -0.06 |
| Week 16 | -0.15 | -0.08 |
| XEOMIN (6-17 years) N = 148 | Placebo (6-17 years) N=72 |
|
|---|---|---|
|
||
| Week 4* | 0.91 | 0.63 |
| Week 8 | 0.94 | 0.54 |
| Week 12 | 0.87 | 0.47 |
| Week 16 | 0.77 | 0.38 |
Efficacy in pediatric patients 2 to 5 years of age is extrapolated from the finding of efficacy in older pediatric patients.
14.2 Upper Limb Spasticity
Upper Limb Spasticity in Adult Patients
The efficacy and safety of XEOMIN for the treatment of upper limb spasticity in adult patients were evaluated in two Phase 3, randomized, multi-center, double-blind studies.
Study 1 and Study 2 were both prospective, double-blind, placebo-controlled, randomized, multi-center trials with an open-label extension period (OLEX) to investigate the efficacy and safety of XEOMIN in the treatment of post-stroke spasticity of the upper limb. For patients who had previously received botulinum toxin treatment in any body region, Study 1 and Study 2 required that ≥12 months and ≥4 months, respectively, had passed since the most recent botulinum toxin administration.
Study 1 consisted of a 12-week main phase followed by three 12-week OLEX treatment cycles for a total exposure duration of 48 weeks. The study included 317 treatment-naïve patients who were at least three months post-stroke in the main study period (210 XEOMIN and 107 placebo). During the main period, XEOMIN (fixed total dose of 400 Units) and placebo were administered intramuscularly to the defined primary target clinical pattern chosen from among the flexed elbow, flexed wrist, or clenched fist patterns and to other affected muscle groups. 296 treated patients completed the main phase and participated in the first OLEX cycle. Each OLEX cycle consisted of a single treatment session (XEOMIN 400 Units total dose, distributed among all affected muscles) followed by a 12-week observation period.
Study 2 consisted of a 12-to-20-week main phase followed by an OLEX period of 48 – 69 weeks, for up to 89 weeks of exposure to XEOMIN. The study included 148 treatment-naïve and pre-treated patients with a confirmed diagnosis of post-stroke spasticity of the upper limb who were at least six months post-stroke (73 XEOMIN and 75 placebo). During the main period, for each patient, the clinical patterns of flexed wrist and clenched fist were treated with fixed doses (90 Units and 80 Units, respectively). Additionally, if other upper limb spasticity patterns were present, the elbow, forearm and thumb muscles could be treated with fixed doses of XEOMIN per muscle. 145 patients completed the main phase and participated in the OLEX period, during which time the dosing of each involved muscle could be adapted individually. During the main and OLEX periods, the maximum total dose per treatment session and 12-week interval was 400 Units.
The average XEOMIN doses injected into specific muscles and the number of injection sites per muscle in Study 1 and Study 2 are presented in Table 18.
| Muscle Group | Muscle | Study 1 Units Injected | Injection Site Per Muscle | Study 2 Units Injected | Injection Site Per Muscle |
|---|---|---|---|---|---|
| XEOMIN (N=210) Mean±SD | XEOMIN Median (Min; Max) | XEOMIN (N=73) Mean±SD | XEOMIN Median (Min; Max) |
||
| All | Overall | 400 ± 2 Units | -- | 307 ± 77 Units | -- |
| Elbow flexors | Overall | 151 ± 50 Units | 5 (1; 11) | 142 ± 30 Units | 5 (2; 9) |
| Biceps | 90 ± 21 Units | 3 (1; 4) | 80 ± 0 Units | 3 (2; 4) | |
| Brachialis | 52 ± 26 Units | 2 (1; 4) | 50 ± 0 Units | 2 (1; 2) | |
| Brachioradialis | 43 ± 16 Units | 2 (1; 3) | 60 ± 2Units | 2 (1; 3) | |
| Wrist flexors | Overall | 112 ± 43 Units | 4 (1; 6) | 90 ± 0 Units | 4 (4; 4) |
| Flexor carpi radialis | 58 ± 22 Units | 2 (1; 3) | 50 ± 0 Units | 2 (2; 2) | |
| Flexor carpi ulnaris | 56 ± 22 Units | 2 (1; 3) | 40 ± 0 Units | 2 (2; 2) | |
| Finger flexors | Overall | 104 ± 35 Units | 4 (1; 4) | 80 ± 0 Units | 4 (4; 4) |
| Flexor digitorum profundus | 54 ± 19 Units | 2 (1; 2) | 40 ± 0 Units | 2 (2; 2) | |
| Flexor digitorum superficialis | 54 ± 19 Units | 2 (1; 2) | 40 ± 0 Units | 2 (2; 2) | |
| Forearm pronators | Overall | 52 ± 24 Units | 2 (1; 3) | 47 ± 16 Units | 2 (1; 3) |
| Pronator quadratus | 26 ± 13 Units | 1 (1; 1) | 25 ± 0 Units | 1 (1; 1) | |
| Pronator teres | 42 ± 13 Units | 1 (1; 2) | 40 ± 0 Units | 1.5 (1; 2) | |
| Thumb flexors/ adductors | Overall | 37 ± 25 Units | 2 (1; 4) | 25 ± 10 Units | 1.5 (1; 3) |
| Adductor pollicis | 14 ± 8 Units | 1 (1; 1) | 10 ± 0 Units | 1 (1; 1) | |
| Flexor pollicis brevis / opponens pollicis | 14 ± 9 Units | 1 (1; 1) | 10 ± 0 Units | 1 (1; 1) | |
| Flexor pollicis longus | 26 ± 16 Units | 1 (1; 2) | 20 ± 0 Units | 1 (1; 1) |
In Study 1, the primary efficacy variable was the change from baseline in Ashworth Scale (AS) score of the primary target clinical pattern determined by the investigator at the Week 4 visit. The Ashworth Scale is a clinical measure of the severity of spasticity by judging resistance to passive movement. The spasticity of the elbow flexors, wrist flexors, finger flexors, and thumb muscles as well as the forearm pronators was assessed on the 0 to 4-point Ashworth scale at each visit. The co-primary efficacy variable of Study 1 was the Investigator's Global Impression of Change Scales (GICS) after 4 Weeks of treatment with XEOMIN or placebo. The GICS is a global measure of a subject's functional improvement. Investigators were asked to evaluate the subject's global change in spasticity of the upper limb due to treatment, compared to the condition before the last injection. The response was assessed using a 7-point Likert scale that ranges from –3 (very much worse) to +3 (very much improved). XEOMIN was considered to be superior to placebo in Study 1 only if statistical significance was reached in both the AS and GICS variables.
The primary efficacy results are displayed in Table 19.
| Mean Change in Ashworth Scale | ||
|---|---|---|
| XEOMIN (N=171) | Placebo (N=88) |
|
| The analysis is based on Last Observation Carried Forward in the Intent To Treat population. p<0.001 | ||
| Total Primary Target Clinical Pattern (flexed wrist, flexed elbow, and clenched fist) | -0.9 | -0.5 |
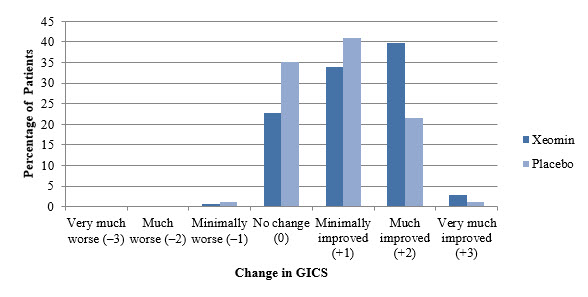
A greater percentage of XEOMIN-treated subjects (43%) than placebo-treated subjects (23%) reported 'very much improved' and 'much improved' in their spasticity (see Figure 8).
Figure 8: Investigator's GICS in Adult Upper Limb Spasticity Study 1
Upper Limb Spasticity in Pediatric Patients
Study 1 was a prospective, double-blind, dose-response, randomized, multi-center trial with an open-label extension period to evaluate the efficacy and safety of XEOMIN for the treatment of upper limb spasticity in pediatric patients. Study 1 enrolled a total of 350 pediatric patients 2 to 17 years of age with upper limb spasticity in one or both upper limbs. In the double-blind main period of Study 1, patients were randomized to one of three dosages of XEOMIN: 2 Units/kg (maximum 50 Units per upper limb), 6 Units/kg (maximum 150 Units per upper limb); or 8 Units/kg (maximum 200 Units per upper limb). The maximum dose, if both upper limbs were treated, respectively was 4 Units/kg (maximum 100 Units), 12 Units/kg (maximum 300 Units), or 16 Units/kg (maximum 400 Units). For treatment of flexed elbow, injection of biceps brachii was mandatory. The investigator could select 1 of the 2 other muscles contributing to spasticity of elbow flexion (i.e., brachialis and brachioradialis) for injection. For patients needing treatment for a flexed wrist, both the flexor carpi radialis and flexor carpi ulnaris were injected. Study 1 used a dose-response design, in which the two highest dosages of XEOMIN (8 Units/kg and 6 Units/kg) were compared to the lowest dosage (2 Units/kg), which served as control. In the absence of a placebo control, the efficacy of the 2 Units/kg dosage of XEOMIN could not be evaluated in Study 1.
The co-primary efficacy variables in Study 1 were the change from baseline on the Ashworth Scale for the primary clinical target pattern (i.e., elbow flexors or wrist flexors), and the Investigator's Global Impression of Change Scale (GICS), both at Week 4. The GICS is a global measure of a subject's functional improvement based on a 7-point Likert scale that ranges from -3 = very much worse to +3 = very much improved.
As displayed in Table 20, the change from baseline in Ashworth Scale score was significantly greater for patients treated with XEOMIN 8 Units/kg than for patients treated with XEOMIN 2 Units/kg. The difference in GICS score between patients treated with XEOMIN 8 Units/kg and those treated with XEOMIN 2 Units/kg did not reach statistical significance. However, the clinical meaningfulness of the difference in Ashworth Scale score change between patients treated with XEOMIN 8 Units/kg and those treated with XEOMIN 2 Units/kg was established by a responder analysis, in which the proportion of patients with a 1-point change or greater on the Ashworth Scale was examined. In that analysis, 86% of patients treated with XEOMIN 8 Units/kg met the responder definition, compared to 71% of patients treated with XEOMIN 2 Units/kg (nominal p value = 0.0099).
There was no significant difference in change from baseline in Ashworth Scale score, GICS score, or proportion of responders between patients treated with XEOMIN 6 Units/kg and those treated with XEOMIN 2 Units/kg. Therefore, the efficacy of a 6 Units/kg dosage of XEOMIN for the treatment of upper limb spasticity in pediatric patients was not established in Study 1.
| XEOMIN 2 Units/kg (N=87) | XEOMIN 8 Units/kg (N=176) |
|
|---|---|---|
| LS = Least Square Mean difference CI = Confidence Interval |
||
|
||
| Ashworth Scale | ||
| Mean Change from Baseline at Week 4 | -0.9 | -1.2 |
| LS Mean Difference versus XEOMIN 2 Units/kg (95% CIs) | -- | -0.22*
(-0.40, -0.04) |
| GICS | ||
| Mean at Week 4 | 1.6 | 1.7 |
| LS Mean Difference versus XEOMIN 2 Units/kg (95% CIs) | -- | 0.09 (-0.10, 0.28) |
14.3 Cervical Dystonia
XEOMIN has been investigated in a randomized, double-blind, placebo-controlled, multicenter trial in a total of 233 patients with cervical dystonia. Patients had a clinical diagnosis of predominantly rotational cervical dystonia, with baseline Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) total score ≥20, TWSTRS severity score ≥10, TWSTRS disability score ≥3, and TWSTRS pain score ≥1. For patients who had previously received a botulinum toxin treatment for cervical dystonia, the trial required that ≥10 weeks had passed since the most recent botulinum toxin administration. Patients with swallowing disorders or any significant neuromuscular disease that might interfere with the study were excluded from enrollment. Patients were randomized (1:1:1) to receive a single administration of XEOMIN 240 Units (n=81), XEOMIN 120 Units (n=78), or placebo (n=74). Each patient received a single administration of 4.8 mL of reconstituted study agent (XEOMIN 240 Units, XEOMIN 120 Units, or placebo). The investigator at each site decided which muscles would receive injections of the study agent, the number of injection sites, and the volume at each site. The muscles most frequently injected were the splenius capitis/semispinalis, trapezius, sternocleidomastoid, scalene, and levator scapulae muscles. Table 21 indicates the average XEOMIN dose, and percentage of total dose, injected into specific muscles in the pivotal clinical trial.
| XEOMIN Dose Injected | |||
|---|---|---|---|
| Number of Patients Injected Per Muscle | Median XEOMIN Units | 75th percentile XEOMIN Units | |
| Sternocleidomastoid | 63 | 25 | 35 |
| Splenius capitis/ Semispinalis capitis | 78 | 48 | 63 |
| Trapezius | 55 | 25 | 38 |
| Levator scapulae | 49 | 25 | 25 |
| Scalenus (medius and anterior) | 27 | 20 | 25 |
Most patients received a total of 2-10 injections into the selected muscles. Patients were assessed by telephone at one week post-injection, during clinic visits at Weeks 4 and 8, and then by telephone assessments or clinic visits every two weeks up to Week 20.
The mean age of the study patients was 53 years, 66% of the patients were women, and 91% were White. At study baseline, 61% of patients had previously received a botulinum toxin as treatment for cervical dystonia. The study was completed by 94% of study patients. Three patients discontinued the study prematurely due to adverse events: two patients in the 240 Unit group experienced musculoskeletal pain and muscle weakness, and one patient in the 120 Unit group experienced nausea and dizziness.
The primary efficacy endpoint was the change in the TWSTRS total score from baseline to Week 4 post-injection, in the intent-to-treat (ITT) population, with missing values replaced by the patient's baseline value. TWSTRS evaluates the severity of dystonia, patient-perceived disability from dystonia, and pain, with a range of possible scores from 0 to 85. The mean change in the total TWSTRS score was significantly greater for both XEOMIN groups than for the placebo group (Table 22).
| TWSTRS Assessment | XEOMIN 240 Units (N=78) | XEOMIN 120 Units (N=81) | Placebo (N =74) |
|---|---|---|---|
| SD = Standard Deviation, CI = Confidence Interval. | |||
|
|||
| Total TWSTRS at baseline | 42.1 | 42.6 | 41.8 |
| Total TWSTRS at Week 4 | 31.2 | 32.7 | 39.5 |
| Mean (SD) Change in TWSTRS score from baseline to Week 4 | -10.9 (11.7) | -9.9 (10.4) | -2.2 (7.3) |
| Mean difference from placebo (95% CI) | -9.0 (-12.0, -5.9) | -7.5 (-10.4, -4.6) | |
| p-value* versus placebo | <0.001 | <0.001 | |
The efficacy of XEOMIN was similar in patients who were botulinum toxin naïve and those who had received botulinum toxin prior to this study.
Examination of age and gender subgroups did not identify differences in response to XEOMIN among these subgroups.
14.4 Blepharospasm
Treatment-Naïve Patients
The efficacy and safety of XEOMIN for the treatment of blepharospasm in treatment-naïve patients were evaluated in Study 1, a randomized, double-blind, placebo-controlled, multi-center trial in a total of 61 patients. Patients had a clinical diagnosis of blepharospasm, with a baseline Jankovic Rating Scale (JRS) severity subscore ≥2. Patients were defined as treatment-naïve if at least 12 months had passed since their last botulinum toxin treatment for blepharospasm. During the placebo-controlled phase, a fixed total dose of 25 Units XEOMIN (n=22), 50 Units XEOMIN (n=19), or placebo (n=20) was administered intramuscularly at 6 injection sites per eye (Figure 6). Of the 61 patients randomized, 55 patients completed the placebo-controlled phase. Patients only continued to the open-label extension (OLEX) period if they had a confirmed need for a re-injection by week 20 of the placebo-controlled phase. A total of 39 patients entered and completed the OLEX phase.
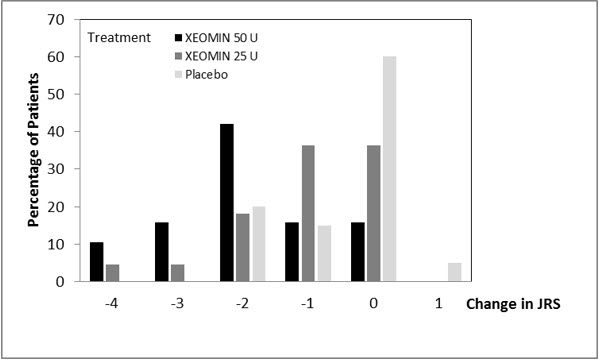
The primary efficacy variable was the change from baseline in JRS Severity subscore determined at Week 6 after the injection. The 50 Unit treatment group demonstrated statistically significant improvements compared to placebo, with a difference of -1.2 (p=0.0004). The change from baseline in the JRS Severity subscore for the 25 Unit treatment group 6 weeks after the injection was not statistically significant, with a difference of -0.5 (p=0.1452) compared to placebo (see Figure 9).
Figure 9: Frequency Distribution of Changes from Baseline JRS Severity Subscore at Week 6 for Treatment-Naïve Patients
Pre-Treated Patients
The efficacy and safety of XEOMIN for the treatment of blepharospasm patients pre-treated with onabotulinumtoxinA (Botox) were evaluated in Study 2, a randomized, double-blind, placebo-controlled, multi-center trial in a total of 109 patients. Patients had a clinical diagnosis of benign essential blepharospasm, with baseline JRS Severity subscore ≥2, and a stable satisfactory therapeutic response to previous administrations of onabotulinumtoxinA (Botox). At least 10 weeks had to have elapsed since the most recent onabotulinumtoxinA administration. Patients with any significant neuromuscular disease that might interfere with the study were excluded from enrollment. Patients were randomized (2:1) to receive a single administration of XEOMIN (n=75) or placebo (n=34). Each patient in the XEOMIN group received a XEOMIN treatment (dose, volume, dilution, and injection sites per muscle) that was similar to the most recent onabotulinumtoxinA injection sessions prior to study entry. The highest dose permitted in this study was 100 Units (50 Units per eye); the mean XEOMIN dose was 33 Units per eye.
In Table 23 the most frequently injected sites, the median dose per injection site, and the median number (and range) of injection sites per eye are presented.
| Injection Area | Median Units XEOMIN | Median Number of Injection Sites (Min-Max) |
|---|---|---|
| Temporal Area | 13 | 2 (1 – 6) |
| Eyebrow Area | 5 | 1 (1 – 4) |
| Upper Lid Area | 10 | 2 (1 – 4) |
| Lower Lid Area | 8 | 2 (1 – 3) |
| Orbital Rim | 5 | 1 (1 – 3) |
Patients were assessed during clinic visits at Weeks 3 and 6, and then by telephone or at clinic visits every two weeks up to Week 20.
The mean age of the study patients was 62 years, 65% of the patients were women, and 83% were White. The study was completed by 94% of study patients. Approximately one third of patients had other dystonic phenomena; in all but 1% this was limited to facial, cervical, perioral and mandibular muscles. No patients discontinued the study prematurely due to adverse events.
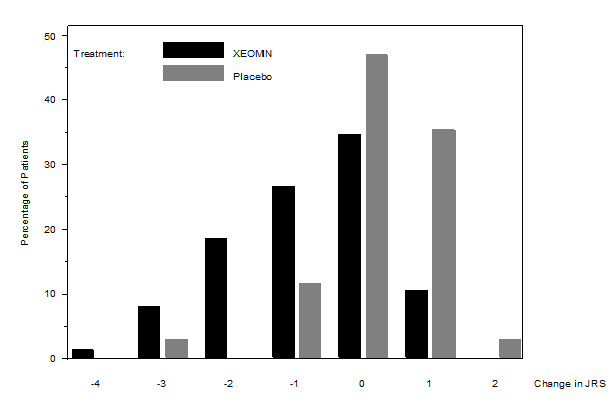
The primary efficacy endpoint was the change in the JRS Severity subscore from baseline to Week 6 post-injection, in the intent-to-treat (ITT) population, with missing values replaced by the patient's most recent value (i.e., last observation carried forward). In the ITT population, the difference between the XEOMIN group and the placebo group in the change of the JRS Severity subscore from baseline to Week 6 was -1.0 (95% CI -1.4; -0.5) points. Comparison of the XEOMIN group to the placebo group was statistically significant at p<0.001.
Figure 10: Frequency Distribution of Changes from Baseline JRS Severity Subscore at Week 6
Examination of age and gender subgroups did not identify substantial differences in response to XEOMIN among these subgroups.
14.5 Glabellar Lines
Two identically designed randomized, double-blind, multi-center, placebo controlled clinical trials (Studies GL-1 and GL-2) were conducted to evaluate XEOMIN for use in the temporary improvement of moderate to severe glabellar lines. The studies enrolled 547 healthy patients (≥18 years old) with glabellar lines of at least moderate severity at maximum frown. Three hundred sixty six subjects were treated with 20 Units of XEOMIN and 181 subjects were treated with placebo. Subjects were excluded if they had marked ptosis, deep dermal scarring, or an inability to lessen glabellar lines, even by physically spreading them apart. The mean age of study subjects was 46 years. The majority of patients were female (86% and 93% in Studies GL-1 and GL-2, respectively), and predominantly Caucasian (89% and 65% respectively). The study subjects received either 20 Units of XEOMIN or an equal amount of placebo. The total dose was delivered in 5 equally divided intramuscular injections of 4 Units each to specific sites (see Figure 7). Subjects were followed up for 120 days.
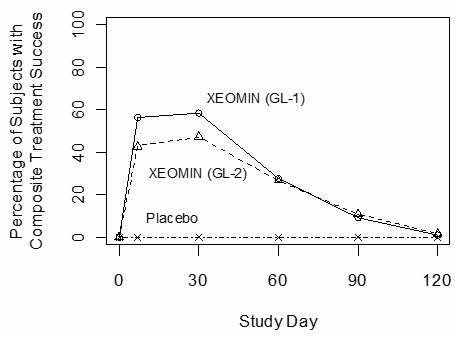
Investigators and subjects assessed efficacy at maximum frown on Day 30 of treatment using a 4-point scale (0=none, 1=mild, 2=moderate, 3=severe). Composite treatment success was defined as a 2-grade improvement on this scale compared to baseline for both the investigator's and subject's assessments on Day 30. The percentage of subjects with treatment success was greater on the XEOMIN arm than the placebo arm at Day 30 in both studies (see Table 24). The percentage of subjects with composite treatment success at each visit is presented in Figure 11.
| GL-1 | GL-2 | |||
|---|---|---|---|---|
| XEOMIN (N=184) | Placebo (N=92) | XEOMIN (N=182) | Placebo (N=89) |
|
|
||||
| Composite Treatment Success* | 111 (60%) | 0 (0%) | 87 (48%) | 0 (0%) |
| Investigator Assessment | 141 (77%) | 0 (0%) | 129 (71%) | 0 (0%) |
| Subject Assessment | 120 (65%) | 0 (0%) | 101 (55%) | 1 (1%) |
Figure 11: Percentage of Subjects with Composite Treatment Success by Visit – Observed Cases (GL-1 and GL-2)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
XEOMIN for injection is a sterile white to off-white lyophilized powder supplied in Type 1 borosilicate glass single-dose vials with tamper-proof aluminum seals and bromobutyl rubber closures that are not made with natural rubber latex in the following pack sizes:
Upper Limb Spasticity and Cervical Dystonia
| Package | XEOMIN 50 Units | XEOMIN 100 Units | XEOMIN 200 Units |
| Carton with one single-dose vial | NDC 0259-1605-01 | NDC 0259-1610-01 | NDC 0259-1620-01 |
16.2 Storage and Handling
Unopened vials of XEOMIN should be stored at or below 25°C (77°F). Refrigeration of unopened vials is not required. Do not use after the expiration date on the vial. Reconstituted XEOMIN may be stored in a refrigerator at 2°C to 8°C (36°F to 46°F) for up to 24 hours until time of use [see Dosage and Administration (2.7)].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Swallowing, Speaking, or Breathing Difficulties or Other Unusual Symptoms
Advise patients to inform their healthcare provider if they develop any unusual symptoms, including difficulty with swallowing, speaking, or breathing, or if any existing symptom worsens [see Boxed Warning and Warnings and Precautions (5.1, 5.4)]. Inform patients of the risk of aspiration.
Ability to Operate Machinery or Vehicles
Counsel patients that if loss of strength, muscle weakness, blurred vision, or drooping eyelids occur, they should avoid driving a car or engaging in other potentially hazardous activities.
Corneal Exposure, Corneal Ulceration, and Ectropion in Patients Treated for Blepharospasm
Inform patients that injections of XEOMIN may cause reduced blinking or effectiveness of blinking, and that they should seek immediate medical attention if eye pain or irritation occurs following treatment [see Warnings and Precautions (5.5)].
Manufactured by:
Merz Pharmaceuticals GmbH
Eckenheimer Landstrasse 100
Frankfurt Germany
U.S. License Number 1830
Distributed by:
Merz Pharmaceuticals, LLC
6601 Six Forks Road, Suite 430
Raleigh, NC 27615
and
Merz North America, Inc.
4133 Courtney Street, Suite 10
Franksville, WI 53126
© 2023 Merz Pharmaceuticals, LLC
XEOMIN® is a registered trademark of Merz Pharma GmbH & Co KGaA.
All trademarks are the property of their respective owners.
Patent www.https://www.merztherapeutics.com/us/patents/
IN00180-01
| This Medication Guide has been approved by the U. S. Food and Drug Administration. | Revised: 9/2023 | |||
| MEDICATION GUIDE XEOMIN® (Zeo-min) (incobotulinumtoxinA) for injection, for intramuscular or intraglandular use |
||||
| What is the most important information I should know about XEOMIN? XEOMIN may cause serious side effects that can be life-threatening. Call your doctor or get medical help right away if you have any of these problems after treatment with XEOMIN:
|
||||
|
|
|||
| These symptoms can happen hours to weeks after you receive an injection of XEOMIN..
These problems could make it unsafe for you to drive a car or do other dangerous activities. See "What should I avoid while receiving XEOMIN?" |
||||
| What is XEOMIN?
XEOMIN is a prescription medicine:
|
||||
Do not take XEOMIN if you:
|
||||
Before receiving XEOMIN, tell your doctor about all of your medical conditions, including if you:
Using XEOMIN with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received XEOMIN in the past. Especially tell your doctor if you:
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine. |
||||
How will I receive XEOMIN?
|
||||
| What should I avoid while taking XEOMIN?
XEOMIN may cause loss of strength or general muscle weakness, blurred vision, or drooping eyelids within hours to weeks of taking XEOMIN. If this happens, do not drive a car, operate machinery, or do other dangerous activities. See "What is the most important information I should know about XEOMIN? " |
||||
| What are the possible side effects of XEOMIN? XEOMIN may cause serious side effects, including: See "What is the most important information I should know about XEOMIN?"
|
||||
|
|
|||
| The most common side effects of XEOMIN in children 2 to 17 years of age with chronic sialorrhea include: | ||||
|
|
|||
| The most common side effects of XEOMIN in adults with upper limb spasticity include: | ||||
|
|
|||
| The most common side effects of XEOMIN in children 2 to 17 years of age with upper limb spasticity include: | ||||
| ||||
| The most common side effects of XEOMIN in adults with cervical dystonia include: | ||||
|
|
|||
| The most common side effects of XEOMIN in adults with blepharospasm include: | ||||
|
|
|||
| The most common side effect of XEOMIN in adults with glabellar lines include: | ||||
| ||||
| These are not all the possible side effects of XEOMIN. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
| General information about the safe and effective use of XEOMIN.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or doctor for information about XEOMIN that is written for health professionals. |
||||
| What are the ingredients in XEOMIN? Active ingredient: botulinum toxin type A Inactive ingredients: human albumin and sucrose Manufactured by: Merz Pharmaceuticals GmbH, Eckenheimer Landstrasse 100, Frankfurt Germany U.S. License Number 1830 Distributed by: Merz Pharmaceuticals, LLC, 6601 Six Forks Road, Suite 430, Raleigh, NC 27615 and Merz North America, Inc. 4133 Courtney Street, Suite 10, Franksville, WI 53126 © 2023 Merz Pharmaceuticals, LLC, XEOMIN® is a registered trademark of Merz Pharma GmbH & Co KGaA. All trademarks are the property of their respective owners. Patent www.https://www.merztherapeutics.com/us/patents/ IN00180-01 |
||||
PRINCIPAL DISPLAY PANEL - 50 Unit Vial Carton
1 vial
Rx Only
NDC 0259-1605-01
incobotulinumtoxinA
XEOMIN®
for Injection
For Intramuscular
and Intraglandular Use
WARNING: Dosing Units of botulinum toxins are
not interchangeable between commercial products.
MERZ
50 units/vial
Dispense the enclosed Medication
Guide to each patient.
PRINCIPAL DISPLAY PANEL - 100 Unit Vial Carton
1 vial
Rx Only
NDC 0259-1610-01
incobotulinumtoxinA
XEOMIN®
for Injection
For Intramuscular
and Intraglandular Use
WARNING: Dosing Units of botulinum toxins are
not interchangeable between commercial products.
MERZ
100 units/vial
Dispense the enclosed Medication
Guide to each patient.

PRINCIPAL DISPLAY PANEL - 50 Unit Vial Carton - NDC 0259-4150-01
1 vial
Rx Only
NDC 0259-4150-01
incobotulinumtoxinA
XEOMIN®
for Injection
For Intramuscular
and Intraglandular Use
WARNING: Dosing Units of botulinum toxins are
not interchangeable between commercial products.
MERZ
50 units/vial
Dispense the enclosed Medication
Guide to each patient.
PRINCIPAL DISPLAY PANEL - 100 Unit Vial Carton - NDC 0259-4110-01
1 vial
Rx Only
NDC 0259-4110-01
incobotulinumtoxinA
XEOMIN®
for Injection
For Intramuscular
and Intraglandular Use
WARNING: Dosing Units of botulinum toxins are
not interchangeable between commercial products.
MERZ
100 units/vial
Dispense the enclosed Medication
Guide to each patient.

PRINCIPAL DISPLAY PANEL - 200 Unit Vial Carton
1 vial
Rx Only
NDC 0259-1620-01
incobotulinumtoxinA
XEOMIN®
for Injection
For Intramuscular
and Intraglandular Use
WARNING: Dosing Units of botulinum toxins are
not interchangeable between commercial products.
MERZ
200 units/vial
Dispense the enclosed Medication
Guide to each patient.
